16
Patients with Temporomandibular Disorders
Introduction
Classification and epidemiology
Diagnostic procedures
Axis I
Assessment of pain
Axis II
Risk factors and etiology
Pathophysiology
Management
Physical management
Pharmacological management
Psychological management
Surgical management
Summary
References
Introduction
Essentially, there are two distinct views on how orthodontics and temporomandibular disorders (TMD) relate to each other: a traditional view holds that abnormal occlusal traits impinge on the function of the jaw muscles and the temporomandibular joint (TMJ) leading to ‘dysfunctional’ movements and abnormal muscle recruitment, which then causes pain in the overloaded tissue. From this a logical approach will be to correct the malocclusion, for example, by orthodontic means. The second and opposite view challenges this relationship and basically disconnects occlusal traits from any relationship to TMD pain problems. Can both views be wrong or right? Controversies should not be clouded by personal beliefs, emotions, traditions or professional politics, but rather resolved by critical examination of the evidence in favor or against the hypothesis. The present chapter attempts to summarize what is known about TMD pain problems with implications for orthodontics, and will also point out significant questions and issues which may not be answered or guided by the highest level of evidence yet, but where patient-based concerns and clinical experience must be used in the prudent management of individual patients.
Classification and Epidemiology
The definition and terminology of TMD pain continues to be debated even more than six decades after Costen’s original description of a pain syndrome involving the TMJ. Problems with pain and soreness in the jaw-muscles, noises such as clicking and crepitation in the TMJ and difficulties in moving the jaw – usually restrictions in opening capacity – as well as signs of dental attrition and oral habits with tooth-grinding or tooth-clenching have in the past been lumped into many different categories and labeled various names such as ‘myofascial pain dysfunction’, ‘temporomandibular joint dysfunction’ ‘craniomandibular disorders’, ‘oromandibular disorders’ and bruxism. Different disciplines and professional groups tend to favor their own classification systems, which basically are based on clusters of different signs and symptoms. This may seem a trivial point but is nonetheless also important because the many different classification systems have contributed to difficulties in making comparisons between studies and have hampered the communication between clinicians and researchers.
There is therefore an urgent need to apply a common universal classification system for TMD problems. Although the pathophysiological mechanisms may not be sufficiently understood at this point in time (see later) and there may be no perfect way to classify TMD pain, a non-hierarchical classification scheme based on operationalized and systematic criteria has been developed and extensively tested for reliability and validity (Dworkin and LeResche 1992; Pehling et al. 2002; John et al. 2005). These research diagnostic criteria (RDC/TMD) (Box 16.1) have indeed been a starting point for international collaborations and new clinical research in TMD pain and can also rationally be applied in daily clinical practice.
Box 16.1 Research Diagnostic Criteria for Temporomandibular Disorders (after Dworkin and LeResche 1992)
Axis I: Biomedical/physical status
Muscle diagnoses
- Myofascial pain
- Myofascial pain with limited opening
Disk displacements
- Disk displacement with reduction
- Disk displacement without reduction, with limited opening
- Disk displacement without reduction, without limited opening
Arthralgia, arthritis, arthrosis
- Arthralgia
- Osteoarthritis of the TMJ
- Osteoarthrosis of the TMJ
Axis II: Pain-related disability and psychological status
Graded chronic pain score
- Grade 0 = no TMD pain in the prior 6 months
- Grade I = Low disability – low intensity pain
- Grade II = Low disability – high intensity pain
- Grade III = High disability – moderately limiting
- Grade IV = High disability – severely limiting
Depression after SCL-90-R depression and vegetative symptom scales
- Normal
- Moderate
- Severe
As the name implies the RDC/TMD is not considered static but may, indeed, need modification when new appropriate research data have been collected or when more knowledge is available on the etiological factors and underlying pathophysiology. Furthermore, diagnosis of, for example, muscle contracture, muscle spasm, myositis and other less frequent painful conditions in the masticatory muscles is not possible in this classification scheme. In a similar way various mechanical problems in the TMJ, for example, ankylosis, disk–condyle derangements, hypermobility, and capsular fibrosis have not been included in the RDC/TMD mainly because such conditions are rare (Stegenga 2001). The lack of generally accepted diagnostic criteria for TMD is most likely the reason why epidemiological surveys have provided quite different estimates of prevalence and incidence (Carlsson and LeResche 1995; LeResche 1997).
The descriptive epidemiology has indicated that between 3% and 15% of the population will qualify for a TMD pain diagnosis (LeResche 1997; Drangsholt and LeResche 1999). Few studies have tried to separate TMJ pain from myofascial TMD pain but the latter appears to be less prevalent than the former (Drangsholt and LeResche 1999). Most studies have, however, found that TMD pain is 1.5–2 times more prevalent in women but it is critical to distinguish between the number of TMD cases presenting in the clinic and the number of TMD cases in the community, because treatment seeking patterns and use of health services may bias a biological sex difference (Warren and Fried 2001; Svensson and Sessle 2004). The prevalence of TMD across the lifetime is still debated but there seems to be a peak around 20–45 years for women although elderly people may also experience TMD pain (Riley et al. 1998; Schmitter et al. 2005a). For some types of TMD problems, such as osteoarthrosis there seems to be an increase over the lifespan. There are few good studies on the incidence of TMD problems (i.e. number of new TMD cases per time unit usually per year). There is some evidence that the incidence is in the range of 2–4% with the persistent types being around 0.1% (Drangsholt and LeResche 1999).
Knowledge about the epidemiology of TMD is essential for clinicians to have in mind because it also provides clues about the natural progression of the disorder. Some longitudinal studies have shown substantial variations in the time course of myofascial TMD (Rammelsberg et al. 2003), with 31% being persistent over a 5-year period, 33% being remittent and 36% recurring. Asymptomatic clicks in the TMJ (disk displacement with reduction – DDwR) are very common (10–35%) (Kononen et al. 1996; LeResche 1997) but have been shown very rarely to progress to disk displacement without reduction (DDwoR); in fact none of the 114 adolescents who were followed over a 9-year period progressed from DDwR to DDwoR (Kononen et al. 1996). Interestingly, this study also indicated major fluctuation in the presence and absence of a DDwR so that only 2% of the examined population had a consistent click at all examination points during the 9-year study period (Kononen et al. 1996). This strongly indicates that asymptomatic DDwR should be managed by conservative techniques. Other studies have shown that patients with combined diagnosis of DDwR and arthralgia may have a higher risk of progressing to a DDwoR (Lundh et al. 1987; Sato et al. 2003). Careful considerations of the treatment options are mandatory in this situation but generally the least invasive technique should be chosen, since the long-term outcome of more extensive procedures including prosthodontics, orthodontics and surgery is questionable (see later).
It is a common clinical experience that ethnic and cultural factors may influence the presentation of TMD problems but so far these issues have not received much attention, although it appears that psychological status and pain-related disability (axis II findings) are more likely to vary between races and ethnic groups than the physical TMD status (axis I findings) (Moore and Brødsgaard 1999; Plesh et al. 2005; Reiter et al. 2006). Estimates from current studies suggest that 3–4% of the Western population may demand management of a TMD problem (Magnusson et al. 2002), making this type of functional problem significant to consider in contemporary oral rehabilitation.
Diagnostic Procedures
Axis I
At this stage it is appropriate to consider in more details the proposed RDC/TMD questionnaire and examination form. Readers can find a complete manual of the procedures on the internet website www.rdc-tmdinternational.org and can for free download the questionnaires and examination forms, which are currently available in 17 languages. For an experienced examiner, the physical examination of the patient for potential TMD problems may take no more than 10–12 minutes. The specific items in the examination form may not differ substantially from other forms except that the conditions, for example, how the patient is sitting, exactly where the palpation is done, how much pressure is applied, how many times the open–close movements are repeated, all have been carefully described and should be followed exactly by the examiner in order to obtain an accurate and reliable outcome. This may require training and calibration, and recent studies have highlighted the importance of calibration and recalibration (Schmitter et al. 2005b; List et al. 2006). Training videos are also available on the RDC/TMD internet site and interested readers are referred to this.
The strength of the RDC/TMD also lies in that specific diagnostic algorithms have been developed, for example, the diagnosis of myofascial TMD pain according to the RDC/TMD criteria requires that first, the patient has answered yes to the question about ongoing pain (questionnaire item 3), second, the examiner finds more than three muscle sites out of 20 possible to be painful on standardized palpation (examination items 8 and 10), and, finally, that at least one of these three sites are located on the same side as the pain complaint (examination item 1). If all these criteria are not met, the patient will not be given a myofascial TMD pain diagnosis in contrast to many other classification systems which would have counted the presence of pain or even tenderness on palpation at just one or two sites as a positive diagnosis. Thus, the RDC/TMD system uses stringent and well-characterized criteria for the most usual types of manifest TMD problems and the extensive translation to many languages help to disseminate the system so that both researchers in the field and clinicians treating patients can benefit from a consensus on the terminology. This is predicted to have major impact on the future understanding of TMD pathophysiology and etiological factors as well as management strategies.
Assessment of Pain
Pain is one of the main characteristics of many of the different types of TMD (myofascial TMD pain, TMJ arthralgia, osteoarthritis) and needs to be evaluated carefully. It is important to note that the RDC/TMD questionnaire and examination form also include quantitative measures of the perceived pain intensity using ratings scales such as the numerical rating scales (NRS) and visual analog scales (VAS). Because a simple VAS may not capture the entire complexity of a patient’s pain experience, it has been suggested that a composite measure such as the characteristic pain intensity (CPI), which is the average of the present, worst and average pain in the last time period, is used (questionnaire items 7–9). Additional VAS scores can provide useful information on both the perceived intensity and unpleasantness of clinical pain conditions because pain intensity mainly reflects the sensory-discriminative component of pain, whereas unpleasantness provides information on the hedonic character of pain, that is, the affective and emotional dimensions of pain. Clinical studies on persistent TMD pain generally indicate that the average pain level measured on a 10 cm VAS with the jaws at rest ranges between 3 cm and 5 cm (Svensson and Graven-Nielsen 2001), probably representing ‘moderate’ pain levels (Collins et al. 1997). The perceived pain intensity of TMD patients is often fluctuating and there can be significant differences between the lowest, the highest and the average pain VAS scores during a week (Maixner et al. 1998) and even between different facial sites (Carlson et al. 1998). Therefore, the use of pain diaries can be helpful to estimate the pain since the perceived pain intensity of TMD patients can change over time simply due to regression to the mean (Whitney and Von Korff 1992). The use of pain diaries is, for example, a cornerstone in the evaluation of headache patients and the International Headache Society classification (Headache Classification Subcommittee of the International Headache Society 2004) uses the intensity dimension of the headache to separate between migraine, which is labeled as ‘strong pain’ whereas tension-type headache more often is described as ‘moderate pain’. It is obvious that many internal as well as external factors can affect the VAS scores of perceived pain intensity in the clinic. Nevertheless, subjective reports of pain are the yardstick of pain measurement (Gracely 2006). Continued efforts should be made to distinguish between the perceived pain intensity in the different TMD subgroups. It should be noted that there are also scales available for children and that there is good evidence that these can be reliably used and provide clinical meaningful data (McGrath and Unruh 2006).
In addition to the intensity of pain, the quality of pain should receive attention. The usual way to describe persistent TMD pain is a ‘deep’, ‘dull’ ‘ache’ sometimes with a ‘boring’, ‘pressing’ or ‘tightening’ type of pain (Okeson 2005). This is in contrast to the superficial types of pain (skin and mucosa) being described as ‘pricking’ and ‘burning’ or neuropathic types of pain described as ‘electrical-like’, paroxysmal or ‘sharp’ and ‘burning’. In order to identify the quality of pain, checklists with adjectives are useful. The McGill Pain Questionnaire (MPQ) was originally introduced as an attempt to provide a detailed description of the quality of pain (Melzack 1975), and has since then become one of the most used pain questionnaires (Melzack and Katz 2006). Several studies have examined the validity of the structure of the MPQ and generally it has an acceptable sensitivity, specificity and reliability. In addition to the qualitative description of pain, the MPQ also provides quantitative measures of the different dimensions of pain, that is, pain rating indices of the sensory, affective, evaluative and miscellaneous dimension of pain can be calculated.
In a sample of 200 patients with persistent facial pain including TMD pain, T rp et al. (1997) found that the words ‘aching’, ‘tight’, ‘throbbing’, ‘tender’ ‘exhausting’, ‘nagging’, ‘sharp’ and ‘tiring’ were used by more than 30% of the population. The choice of ‘radiating’ (26%) and ‘pressing’ (22%) seems rather specific for TMD pain conditions compared with other pain conditions (T
rp et al. (1997) found that the words ‘aching’, ‘tight’, ‘throbbing’, ‘tender’ ‘exhausting’, ‘nagging’, ‘sharp’ and ‘tiring’ were used by more than 30% of the population. The choice of ‘radiating’ (26%) and ‘pressing’ (22%) seems rather specific for TMD pain conditions compared with other pain conditions (T rp et al. 1997). Patients with myofascial TMD pain also frequently choose ‘annoying’ (Stohler and Lund 1995). The quality of pain appears to be markedly different between patients with myofascial TMD pain and pain in the TMJ (Mongini and Italiano 2001). It is unclear whether this difference can be explained by neurobiological factors such as activation of different nociceptive fibers in the muscle and joint tissue or may be due to higher order cognitive-emotional differences. Thus, there are certain words which seem to be specifically related to the description of persistent TMD pain, but no word is on the other hand pathognomonic for this pain condition in a similar way that ‘pulsating’ has been tied to migraine and ‘pressing’ and ‘tightening’ to tension-type headache (Headache Classification Subcommittee of the International Headache Society 2004).
rp et al. 1997). Patients with myofascial TMD pain also frequently choose ‘annoying’ (Stohler and Lund 1995). The quality of pain appears to be markedly different between patients with myofascial TMD pain and pain in the TMJ (Mongini and Italiano 2001). It is unclear whether this difference can be explained by neurobiological factors such as activation of different nociceptive fibers in the muscle and joint tissue or may be due to higher order cognitive-emotional differences. Thus, there are certain words which seem to be specifically related to the description of persistent TMD pain, but no word is on the other hand pathognomonic for this pain condition in a similar way that ‘pulsating’ has been tied to migraine and ‘pressing’ and ‘tightening’ to tension-type headache (Headache Classification Subcommittee of the International Headache Society 2004).
Pain from deep structures (muscle, joint, tendon, ligaments) is frequently described as diffuse and difficult to locate precisely in contrast to superficial types of pain (Mense 1993; Svensson and Sessle 2004). Thus, the perceived localization of deep pain may be quite different from the original source of pain. This can also cause problems when TMJ pain must be differentiated from myofascial TMD pain. Pain located to the source of pain is termed local pain whereas pain felt in a different region and/or structure away from source of pain is termed referred pain. The International Association for the Study of Pain (IASP) task force on taxonomy has not provided any official definition of referred pain but in the orofacial region referred pain has been described as pain in other structures and completely separated from the local pain areas (Stohler and Lund 1994). However, the validity of this definition has not been established and there could be a time- or intensity-dependent relationship between local pain in the masticatory muscles, referral of pain and spreading of pain (Svensson and Arendt-Nielsen 2000). This still needs to be studied in more detail. From a practical point of view, the RDC/TMD emphasizes that the examiner points to the TMJ and jaw muscles in order to help the patient determine the source of the pain. TMJ arthralgia is similar to myofascial TMD pain based on both a patient report of ongoing pain in the TMJ (examination item 2) or pain on opening (examination items 4b-c) or on excursion (examination items 6a-b) in addition to pain on palpation (examination items 9a-b). However, other functional tests (e.g. joint play, compression and provocation tests) could in the future prove to be a valuable addition to distinguish between TMJ arthralgia and myofascial TMD pain (Bumann and Lotzmann 2002).
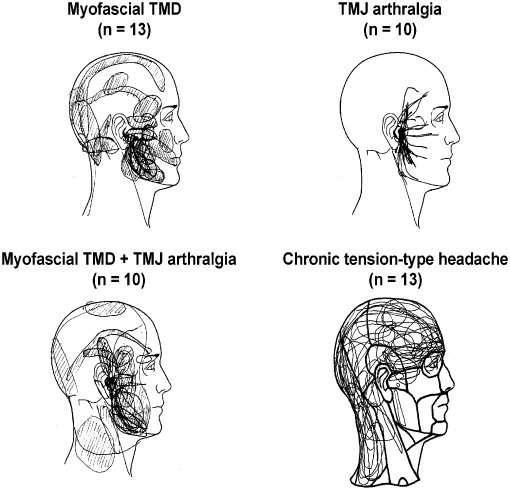
Pain drawings made by the patients are simple, but yet useful tools to illustrate the localization and extent of pain areas in general. Pain drawings administered on a systematic basis to patients with pain complaints in the craniofacial region have revealed that only about 20% have pain confined to this region, whereas 66% have widespread pain outside the craniofacial and cervical regions (T rp et al. 1998). Information on these concomitant sites of pain in other parts of the body is important because they could indicate comorbidity or involvement of more widespread pathophysiological mechanisms in some patients with TMD pain (Dao et al. 1997; John et al. 2003). Alternatively, the reports of pain outside the craniofacial region could to some extent be due to referral of pain (see later). In any case, clinicians should not only use drawings of the head and face but should also obtain a general map of other possible pain conditions in the body. Figure 16.1 shows an example of these maps applied to patients with different RDC/TMD diagnoses as well as tension-type headaches for a comparison.
rp et al. 1998). Information on these concomitant sites of pain in other parts of the body is important because they could indicate comorbidity or involvement of more widespread pathophysiological mechanisms in some patients with TMD pain (Dao et al. 1997; John et al. 2003). Alternatively, the reports of pain outside the craniofacial region could to some extent be due to referral of pain (see later). In any case, clinicians should not only use drawings of the head and face but should also obtain a general map of other possible pain conditions in the body. Figure 16.1 shows an example of these maps applied to patients with different RDC/TMD diagnoses as well as tension-type headaches for a comparison.
Fig. 16.1 Patient-derived pain drawings showing characteristic patterns in patients with myofascial temporomandibular disorder (TMD) pain, temporomandibular joint (TMJ), arthralgia, combined myofascial TMD pain and TMJ arthralgia and chronic tension-type headache. Note the difference between the presentation of the TMD pain and tension-type headache.
In summary, the key points for the diagnostic TMD procedures are to obtain information on the pain intensity using VAS or similar ratings scales and to evaluate pain quality and pain distribution using the MPQ or similar instruments. The physical examination can reliably and rationally be performed by using the RDC/TMD.
Axis II
In addition to the operationalized criteria for the clinical examination of the jaw muscles and TMJs, which form the basis for the so-called axis I or physical diagnosis, the RDC/TMD system is remarkable in the sense that it also provides a tool to examine the consequences of pain-related disability and psychosocial distress (axis II) (Box 16.1). Most clinicians dealing with TMD patients have experienced these psychological alterations, for example, patients seem to be depressed and with many other complaints, and will unfortunately often label such patients as ‘psychogenic’ or chronic pain patients. Nevertheless, the current view on persistent pain conditions including TMD pain problems suggests that these psychological and behavioral changes could be secondary to a long period with pain and not necessarily the cause of the pain. In most cases it will also be impossible to identify an exact cause–effect relationship and it may be more prudent to accept that pain as defined by the IASP has emotional and cognitive-behavioral components.
The RDC/TMD offers the possibility for the clinicians not specifically trained in clinical psychology to evaluate the amount of psychosocial distress on the second axis in the diagnostic system. Thus, the CPI (see above) is linked to a disability score based on specific questions in the history form (questionnaire items 11–13). Based on the CPI and disability points, a 0–4 graded chronic pain scale is created (Box 16.1). The rule of thumb is that dentists in primary care should be trained to diagnose and manage graded chronic pain scale group 1 and group 2 patients whereas specialist training and collaboration with general physicians, psychologists, psychiatrists, pain specialists and pain clinics can be recommended for group 3 and group 4 patients. It is important early to realize the involvement of psychosocial issues and adjust treatment plans and prognosis accordingly and not view psychosocial issues as a last resort when conventional therapy has failed. There is clear evidence that recognition of pain-related disability and psychosocial factors plays an important role in outcome of management and prognosis (Dworkin et al. 2002a,b; Turner and Dworkin 2004). In a similar way, quality of life measures should always be considered and the Oral Health Impact Profile (OHIP) is one well-characterized tool to assess these dimensions (Slade and Spencer 1994; Larsson et al. 2004). Also the RDC/TMD includes some basic assessment of the extent to which oral functions are influenced by pain (questionnaire item 19).
Finally, the issue of depression and somatization is dealt with in a modified psychometric tool (symptom check list, SCL) in the RDC/TMD (questionnaire item 20) and provides the clinicians with important information in terms of referrals and management plan. This approach with early characterization of the pain not only in a somatic manner (axis I) but also in a more comprehensive biopsychosocial way (Suvinen et al. 2005) should be the yardstick for modern oral rehabilitation. It needs to be said that oral rehabilitation must be viewed as more than the physical reconstruction of the occlusion but clearly also involves other biomedical and psychological approaches to bring or restore to a normal or optimal state of health.
Risk Factors and Etiology
Modern epidemiology is far more advanced than a basic description of the prevalence and incidence of TMD symptoms and can also be used as an analytic tool. This line of research has helped to identify a number of factors likely to be related to TMD pain. These factors are termed risk factors to indicate the probability that TMD pain and the factor are related. A stringent view on these risk factors has suggested that very few of the assumed and often clinically believed etiological factors actually meet the criteria for a statistical relationship (Box 16.2).
Box 16.2 Possible Risk Factors for TMD (Modified after Drangsholt and LeResche 1999, Pullinger et al. 1993, Seligman and Pullinger 2000)
Gender/hormonal factors
Depression/somatization
Multiple pain conditions/widespread pain
Bruxism/oral parafunctions
Trauma
Generalized joint hypermobility
Occlusal variables:
- Anterior open bite
- Unilateral crossbite
- Overjet >6–7 mm
- More than 5–6 missing posterior teeth
- RCP–ICP slide >2 mm
- Dental wear
RCP, retruded contact position; ICP, intercuspal contact position.
Note: not all the listed factors are supported by sufficient data to suggest causation.
It must also be noted that although these factors meet or are close to meeting statistical criteria they do not necessarily indicate a straightforward cause-and-effect relationship, which is exemplified by the risk factor depression that conceivably could be an effect of persistent TMD pain rather than its cause. In particular, the occlusal factors have been subject to an overheated and emotional discussion and continue to be within the dental community. A small number of occlusal factors (Box 16.1) appear to be weakly associated with TMD but for many clinicians it is a surprise not to find more occlusal parameters on the list. Again, it must be remembered that a change in occlusion could occur as an effect of an underlying pathology (e.g. osteoarthritis leading to anterior open bite). In particular, many clinicians are surprised not to see the deep bite as a significant risk factor because the conventional view has been that deep bite may lead to a posterior displacement of the mandible, leading to TMJ clicking and degeneration associated with TMJ arthralgia and myofascial TMD pain. This view would at a first glance seem to gain support from a recent report of 320 persons who were followed over 20 years and in which deep bite was a significant risk factor (odds ratio 12.5) for dysfunctional problems (Carlsson et al. 2002). However, a careful reading of the conclusion shows that although there was a significant correlation between deep bite and some signs and symptoms of TMD, the study could not demonstrate that deep bite was a risk factor for manifest TMD pain. In support of this conclusion, another study showed in 3033 persons that deep bite or anterior open bite not was associated with the cardinal signs and symptoms of TMD, that is, pain, limited opening capacity and joint sounds/noises (John et al. 2002).
The current view is that dental occlusion plays only a minor role for development and maintenance of TMD pain and that not more than approximately 25% of the variation of TMD pain can be explained by occlusal factors (Pullinger et al. 1993; McNamara et al. 1995; Seligman and Pullinger 2000; Gesch et al. 2004, 2005). One of the more robust findings is the association between crossbite and TMD (e.g. Sonnesen et al. 1998; Gesch et al. 2004) but further studies will be needed to identify the odds ratio for manifest TMD problems. A challenge for future research is to operationalize and better quantify functional aspects of occlusion since reproducibility and validity of most occlusal examination procedures is poor to modest (Baba et al. 2000). A search for a single etiological factor in the occlusion however, is not warranted (Green 2001), but a better understanding of how dental occlusion and function and other neurobiological/neuromuscular and psychosocial risk factors interact may still be relevant for a complete understanding of orofacial musculoskeletal pain and other localized symptoms from the masticatory system.
From an orthodontic perspective, several studies have suggested that orthodontic treatment neither can cause nor prevent most types of TMD (e.g. Egermark et al. 2003; Henrikson and Nilner 2003; Koh and Robinson 2003; Mohlin et al. 2004), which clearly must be appreciated when the indications for orthodontics are considered (see other chapters in this volume).
Pathophysiology
The exact pathophysiology of TMD pain is not known given the fact that multiple factors related to anatomical, psychological-psychosocial and neurobiological components seem to be involved (De Boever and Carlsson 1994). Thus, TMD may still on a population basis be viewed as a multifactorial condition which in the individual patient actually means an idiopathic pain condition (Green 2001; Svensson and Sessle 2004). Nevertheless, there are several studies in progress to outline some of the potential underlying mechanisms in TMD pain and these will be described briefly in the following paragraphs. For a more detailed review of orofacial pain mechanisms, the reader is referred to recent reviews (e.g. Svensson and Sessle 2004).
One of the most prominent features of painful TMD is the report of pain on palpation of jaw muscles or TMJ. Several studies have indeed reported lower pressure pain thresholds in the jaw muscles of patients with TMD pain compared with normal subjects (Reid et al. 1994; Svensson et al. 1995, 2001; Maixner et al. 1998). The pathophysiological mechanism responsible for lower pain thresholds in deep tissues could be a sensitization of peripheral nociceptors. Animal data have documented that deep noxious inputs cause sensitization of the peripheral receptors (Berberich et al. 1988; Schaible 2006). Thus, endogenous substances released by tissue trauma such as bradykinin, serotonin, prostaglandins, adrenaline, and hypoxia, in addition to the excitatory amino acid glutamate, lower the mechanical threshold of nociceptors into the innoc/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


