Repair and Regeneration of Oral Tissues
Predictive clinical outcomes can be achieved only if biologic aspects of wound healing and tissue repair/regeneration are taken into consideration. In time, the dentist, in pace with other fields of medicine, will routinely use tissue engineering approaches and gene therapy to heal and rebuild oral tissues. A review on the use of biologic therapies has concluded that “craniofacial tissue engineering is likely to be realized in the foreseeable future, and represents an opportunity that dentistry cannot afford to miss” (Mao et al. 2006). As an example, the application of gene therapy to restore salivary gland function is already well advanced, with clinical trials showing very encouraging results.
Wound Healing in Oral Mucosa
Inflammatory Cell Activation, Migration, and Function
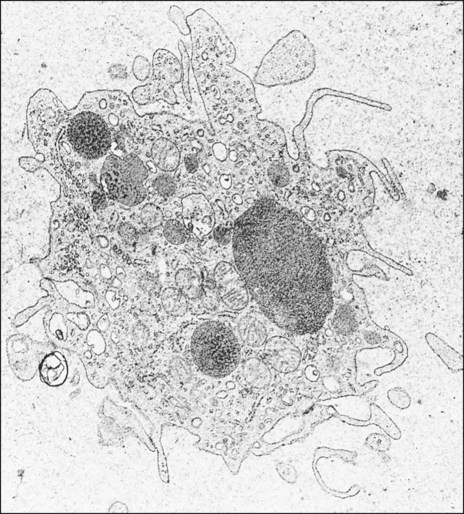
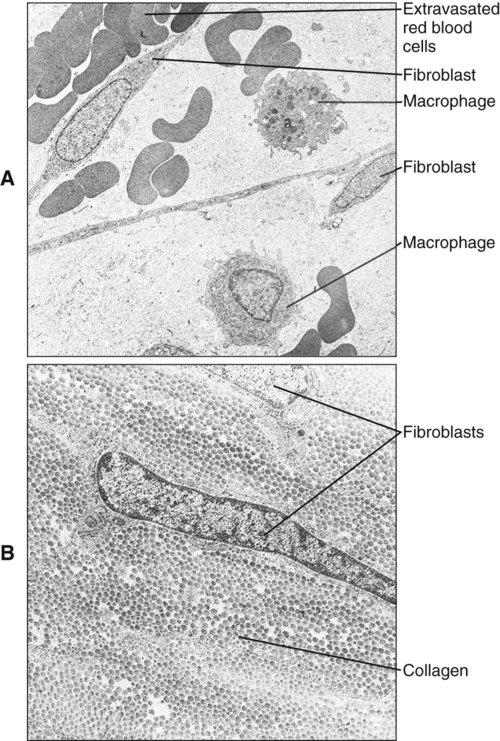
Polymorphonuclear leukocytes, mainly neutrophils, are the first inflammatory cells to invade the wound. They appear within few hours of injury and become activated in response to phagocytic stimuli or by binding of chemotactic mediators, antigen-antibody complexes to specific receptors on the cell membrane, and components of the complement system. These cells reach a maximum concentration at about 24 hours and have a short life span at the wound site before they die. Neutrophils contain various enzymes and reactive oxygen metabolites (oxygen-derived free radicals) that kill engulfed bacteria but that also can destroy damaged and normal tissue when the cells die. Neutrophils function primarily to manage bacterial invasion and hence infection, thus their absence in noninfected wounds does not hinder the repair process. Macrophages and other mononuclear leukocytes enter the wound after 24 hours and are the predominant cell type in damaged tissue at 5 days (Figure 15-1). Macrophage infiltration into the wound site is mediated by various chemotactic factors that are released by platelets in the fibrin clot, keratinocytes at the wound margins, fibroblasts, and leukocytes resulting in cellular and humoral responses and in phagocytosis of damaged tissue components and foreign material. Platelets also release many potent growth factors (transforming growth factor β [TGF-β], platelet-derived growth factor [PDGF], interleukin-1, and others), cytokines, and chemokines. These soluble mediators are critical for the next phase of wound repair involving cell recruitment and differentiation and the commencement of rebuilding damaged tissues. Macrophages are a major source of cytokines involved in lymphocyte chemotaxis and later constitute the most prominent leukocyte subset in wounds. TGF-β in particular stimulates fibroblasts to proliferate and synthesize extracellular matrix proteins. In the absence of macrophages, fewer fibroblasts are stimulated during healing, so that healing is slower.
Reparative Phase
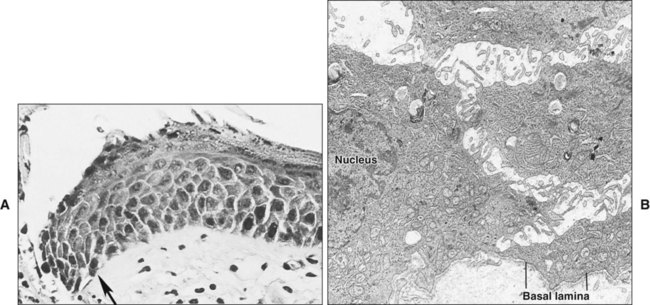
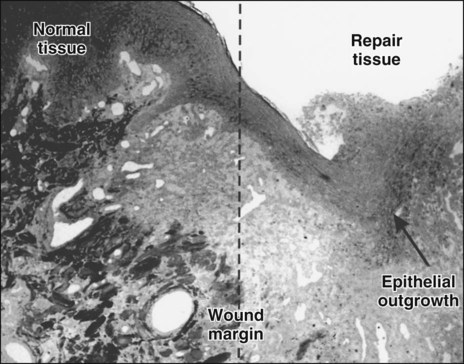
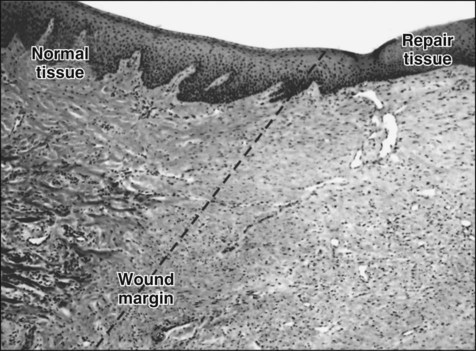
Damage to the epithelium results in mobilization and migration of epithelial cells at the wound margin. The cells lose their close attachment to each other and to the underlying connective tissue within 24 hours of wounding; this is apparent histologically as a widening of the intercellular spaces (Figure 15-2). Twenty-four to 48 hours after wounding, cell division in the basal epithelium increases a short distance behind the wound margin, and those cells immediately adjacent to the margin begin to migrate laterally beneath the clot or coagulum (Figure 15-3). As they migrate, the epithelial cells deposit basal lamina constituents that facilitate movement through the subepithelial connective tissue. Migration and subsequent adhesion of epithelial cells to the basal lamina implicates remodeling of the cytoskeleton and redistribution of integrin membrane receptors, interaction with laminin-332, and ultimately the formation of hemidesmosomes.
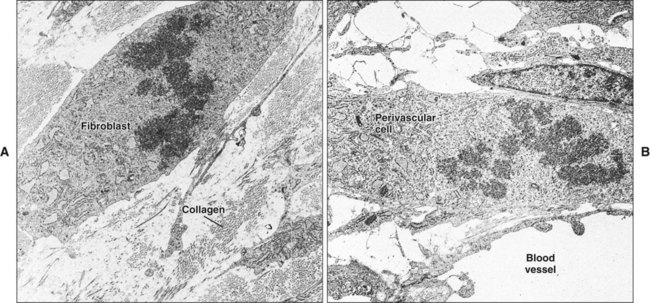
Initially, the wounded connective tissue consists of fibrin, necrotic tissue, and an acute inflammatory cell infiltrate. Fibroblasts migrate and proliferate within the healing connective tissue within 24 hours. The fibroblasts involved in wound repair derive from two sources: division of undamaged fibroblasts at the wound periphery (Figure 15-4, A) and undifferentiated connective tissue (mesenchymal) cells (Figure 15-4, B). The resulting daughter cells from both sources migrate into the wound defect to form the collagen of scar tissue (Figure 15-5). Moreover, endothelial cells proliferate and capillaries develop from preexisting vessels at the wound margin. New blood vessels play an essential role in tissue healing by participating in connective tissue formation, providing nutrients and oxygen, secreting bioactive substances (endothelial cells), and allowing for inflammatory cell migration to the site of injury. Angiogenesis is a complex event regulated by growth factors acting in synergy. Vascular endothelial growth factor, fibroblast growth factor [FGF], and TGF-β are major components in wound angiogenesis. Extracellular matrix molecules, such as fibronectin, laminin, and collagens, are also important in vessel growth by acting as a scaffold for cell migration and reservoir for growth factors.
At 3 days the healing lamina propria is predominantly cellular, consisting of inflammatory cells, developing capillaries, and abundant fibroblasts among fibrin remnants and new collagen fibrils (see Figure 15-3). Between days 5 and 20 after wounding, collagen is deposited rapidly in the wound, with a corresponding increase in tissue tensile strength, although up to 150 days may be required to regain normal tissue strength (Figure 15-6). The relative proportion of cells and fibers approaches that of unwounded tissue by 20 days.
Wound Contraction and Scarring
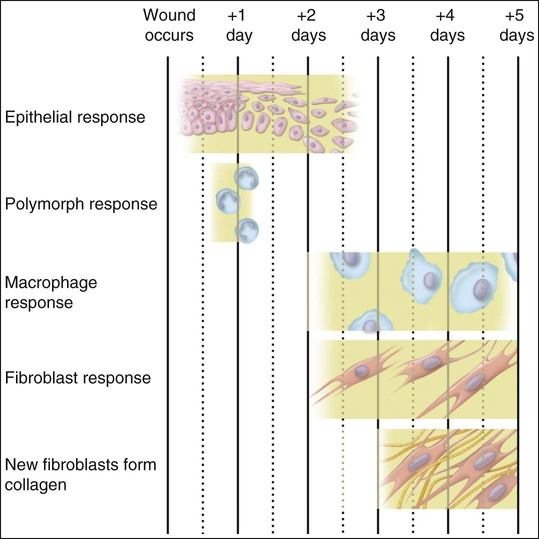
Collagen that is laid down may form scar tissue and lead to rigidity and immobilization of the area, with impairment of function. Myofibroblasts and wound contraction have been described in oral mucosa, but scar tissue that is formed usually is remodeled so that most surgery within the mouth can be undertaken without fear of producing disabling scar tissue. The reason for the differences in wound healing between skin and oral mucosa is not understood, but increasing evidence indicates that fibroblasts in oral mucosa are phenotypically different from those of skin and more closely resemble fetal fibroblasts. Such differences can be seen in the synthesis of glycosaminoglycans and in the response to the cytokine TGF-β. Figure 15-7 provides a summary of this simple account of repair.
Wound Healing at the Dentogingival Junction
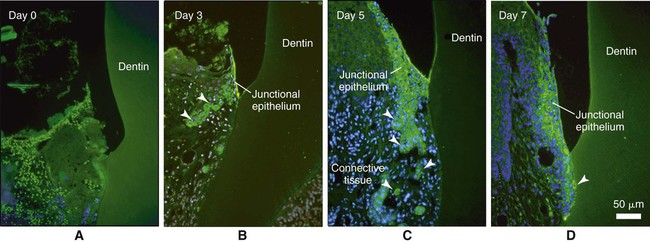
The recent discovery that odontogenic ameloblast–associated (ODAM) protein is produced by the junctional epithelium, and the fact that it is expressed early during its regeneration in animal models (Figure 15-8) implicates this molecule as a potential target for novel prevention and regenerative strategies. ODAM may indeed behave as a matricellular protein influencing both matrix and cellular events.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses