Defense Mechanisms of the Gingiva
Sulcular Fluid
The presence of sulcular fluid, or gingival crevicular fluid (GCF), has been known since the nineteenth century, but its composition and its possible role in oral defense mechanisms were elucidated by the pioneering work of Waerhaug107 and Brill and Krasse13 during the 1950s. The latter investigators introduced filter paper into the gingival sulci of dogs that had previously been injected intramuscularly with fluorescein; within 3 minutes, the fluorescent material was recovered on the paper strips. This indicated the passage of fluid from the bloodstream through the tissues and the exiting of fluid via the gingival sulcus.
In subsequent studies, Brill10,11 confirmed the presence of GCF in humans and considered it a “transudate.” However, others64,108 demonstrated that GCF is an inflammatory exudate rather than a continuous transudate. In strictly normal gingiva, little or no fluid can be collected.
More recently, interest in the development of tests for the detection or prediction of periodontal disease has resulted in numerous research papers about the components, origin, and function of GCF.19
Methods of Collection
The most difficult hurdle to overcome when collecting GCF is the scarcity of material that can be obtained from the sulcus. Many collection methods have been tried.9,12,52,55,66,67,92 These methods include the use of absorbing paper strips, the placement of twisted threads around and into the sulcus, and techniques involving micropipettes and intracrevicular washings.
The absorbing paper strips are placed within the sulcus (intrasulcular method) or at its entrance (extrasulcular method) (Figure 13-1). The placement of the filter paper strip in relation to the sulcus or pocket is important. The Brill technique involves inserting it into the pocket until resistance is encountered (Figure 13-1, A). This method produces some degree of irritation of the sulcular epithelium that by itself can trigger the flow of fluid.
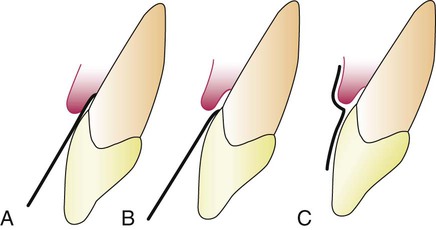
To minimize this irritation, Löe and Holm-Pedersen64 placed the filter paper strip just at the entrance of the pocket or over the pocket entrance (Figure 13-1, B and C). In this way, fluid that seeps out is picked up by the strip, but the sulcular epithelium is not in contact with the paper.
Preweighed twisted threads were used by Weinstein and colleagues.108 The threads were placed in the gingival crevice around the tooth, and the amount of fluid collected was estimated by weighing the sample thread.
The use of micropipettes permits the collection of fluid by capillarity. Capillary tubes of standardized length and diameter are placed in the pocket, and their content is later centrifuged and analyzed.9,11,12
Crevicular washings can be used to study GCF from clinically normal gingiva. One method involves the use of an appliance that consists of a hard acrylic plate that covers the maxilla, with soft borders and a groove that follows the gingival margins; it is connected to four collection tubes. The washings are obtained by rinsing the crevicular areas from one side to the other with the use of a peristaltic pump.20
A modification of the previous method involves the use of two injection needles that have been fitted one within the other so that, during sampling, the inside (ejection) needle is at the bottom of the pocket and the outside (collecting) needle is at the gingival margin. The collection needle is drained into a sample tube via continuous suction.92
Permeability of Junctional and Sulcular Epithelia
The initial studies by Brill and Krasse13 involving the use of fluorescein were later confirmed with substances such as India ink86 and saccharated iron oxide.20 Substances that have been shown to penetrate the sulcular epithelium include albumin,85 endotoxin,84,89 thymidine,43 histamine,23 phenytoin,102 and horseradish peroxidase.70 These findings indicate permeability to substances with a molecular weight of up to 1000 kD.
Squier and Johnson101 reviewed the mechanisms of penetration through an intact epithelium. The intercellular movement of molecules and ions along intercellular spaces appears to be a possible mechanism. Substances that take this route do not traverse the cell membranes.
Amount
An electronic method has been devised for measuring the fluid collected on a “blotter” (Periopaper) with the use of an electronic transducer (Periotron, Harco Electronics, Winnipeg, Manitoba, Canada) (Figure 13-2). The wetness of the paper strip affects the flow of an electronic current and provides a digital readout. A comparison of the ninhydrin-staining method and the electronic method performed in vitro revealed no significant differences between the two techniques.104
The amount of GCF collected is extremely small. Measurements performed by Cimasoni20 showed that a strip of paper 1.5-mm wide and inserted 1 mm within the gingival sulcus of a slightly inflamed gingiva absorbs about 0.1 mg of GCF in 3 minutes. Challacombe18 used an isotope dilution method to measure the amount of GCF present in a particular space at any given time. His calculations for human volunteers with mean gingival indices of less than 1 showed that the mean GCF volume in the proximal spaces from the molar teeth ranged from 0.43 to 1.56 µL.
Composition
The components of GCF can be characterized according to individual proteins,64,76,93 specific antibodies, antigens,30,83 and enzymes of several specificities.14 The GCF also contains cellular elements.23,26,110
Many research efforts have attempted to use GCF components to detect or diagnose active disease or to predict patients who are at risk for periodontal disease.2 So far, more than 40 compounds found in GCF have been analyzed,80 but their origin is not known with certainty. These compounds can be derived from the host or produced by the bacteria in the gingival crevice, but their source can be difficult to elucidate; examples include β-glucuronidase, which is a lysosomal enzyme, and lactic acid dehydrogenase, which is a cytoplasmic enzyme. The sources of collagenases may be fibroblasts or polymorphonuclear leukocytes (PMNs [neutrophils]),4,78 or collagenases may be secreted by bacteria.30 Phospholipases are lysosomal and cytoplasmic enzymes, but they are also produced by microorganisms.14 The majority of GCF elements detected thus far have been enzymes, but there are nonenzymatic substances as well.
Organic Compounds.
Both carbohydrates and proteins have been investigated. Glucose hexosamine and hexuronic acid are two of the compounds that are found in GCF.41 Blood glucose levels do not correlate with GCF glucose levels; glucose concentration in GCF is three to four times greater than that in serum.41 This is interpreted not only as a result of the metabolic activity of adjacent tissues but also as a function of the local microbial flora.
The total protein content of GCF is much less than that of serum.12,13 No significant correlations have been found between the concentration of proteins in GCF and the severity of gingivitis, pocket depth, or extent of bone loss.7
Metabolic and bacterial products identified in GCF include lactic acid,42 urea,36 hydroxyproline,82 endotoxins,98 cytotoxic substances, hydrogen sulfide,100 and antibacterial factors.22
Many enzymes have also been identified.
The methodology used for the analysis of GCF components is as varied as the diversity of those components. Examples include fluorometry for the detection of metalloproteinases23; enzyme-linked immunosorbent assays to detect enzyme levels and interleukin-1β (IL-1β)63; radioimmunoassays for detecting cyclooxygenase derivatives77 and procollagen III105; high-pressure liquid chromatography to detect timidazole59; and direct and indirect immunodot tests for the detection of acute-phase proteins.97
Cellular and Humoral Activity in Gingival Crevicular Fluid
The analysis of GCF has identified cell and humoral responses in both healthy individuals and in those with periodontal disease.56 The cellular immune response includes the appearance of cytokines in GCF, but there is no clear evidence of a relationship between cytokines and disease. However, IL-1α and IL-1β are known to increase the binding of PMNs and monocytes/macrophages to endothelial cells, to stimulate the production of prostaglandin E2 and the release of lysosomal enzymes, and to stimulate bone resorption.60 Preliminary evidence also indicates the presence of interferon-α in GCF,56 which may have a protective role in periodontal disease because of its ability to inhibit the bone resorption activity of IL-1β.38
Because the amount of fluid recoverable from gingival crevices is small, only the use of very sensitive immunoassays permits the analysis of the specificity of antibodies.22,24,25 A study that compared antibodies in different crevices with serum antibodies directed at specific microorganisms did not provide any conclusive evidence regarding the significance of the presence of antibodies in GCF among individuals with periodontal disease.56
Although the role of antibodies in the gingival defense mechanisms is difficult to ascertain, the consensus is that, in a patient with periodontal disease, a reduction in antibody response is detrimental, and an antibody response plays a protective role.57
Clinical Significance
As mentioned previously, GCF is an inflammatory exudate.64 Its presence in clinically normal sulci can be explained, because gingiva that appears clinically normal invariably exhibits inflammation when it is examined microscopically.
The amount of GCF is greater when inflammation is present,29,95 and it is sometimes proportional to the severity of inflammation.79 GCF production is not increased by trauma from occlusion,69 but it is increased by the mastication of coarse foods, toothbrushing and gingival massage, ovulation,63 hormonal contraceptives,62 prosthetic appliances,74 and smoking.71 Other factors that influence the amount of GCF are circadian periodicity and periodontal therapy.
Drugs in Gingival Crevicular Fluid
Drugs that are excreted through the GCF may be used advantageously in periodontal therapy. Bader and Goldhaber6 demonstrated in dogs that tetracyclines are excreted through the GCF; this finding triggered extensive research that showed a concentration of tetracyclines in GCF as compared with serum.37 Metronidazole is another antibiotic that has been detected in human GCF27 (see Chapter 48).
Leukocytes in the Dentogingival Area
Leukocytes have been found in clinically healthy gingival sulci in humans and experimental animals. The leukocytes found are predominantly PMNs. They appear in small numbers extravascularly in the connective tissue adjacent to the bottom of the sulcus; from there, they travel across the epithelium17,39 to the gingival sulcus, where they are expelled (Figures 13-3 and 13-4).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses



