Chapter 11
Problem Solving in Cleaning and Disinfecting the Root Canal System
Problem-solving issues and challenges in cleaning and disinfecting the root canal system addressed in this chapter are:
“One of the most neglected phases of endodontic treatment is the removal of minute fragments of organic debris and dentinal shavings from the root canal. It is an axiomatic principle of surgery that before a wound is ready for chemotherapy, all necrotic material and debris must be removed. Many dentists have failed to appreciate the importance of this basic rule of surgery and have relied principally on drug therapy rather than on thorough cleansing and irrigation of the root canal.”< ?xml:namespace prefix = "mbp" />
The concepts of cleaning and shaping the root canal system have been with us for years. In many respects, the two activities are incompatible when there is sole reliance on intracanal instruments to accomplish these goals. Instruments can shape the canal, but true cleaning relies on irrigants.
What Is the Key Role of and the Rationale for the Use of Intracanal Irrigants?
The key role of root canal irrigants is to clean the canal during the enlarging and shaping process. Specifically, the objectives of the cleaning and shaping process are to remove the vital or necrotic dental pulp tissue and neutralize or eliminate bacteria and their associated metabolic byproducts. Although the shaping of the root canal has been enhanced with advances in metal technology, the actual cleaning of the canal still relies heavily on the adjunctive use of chemical rinses and soaks to achieve these goals because of the anatomic complexity and irregularity of the tooth (
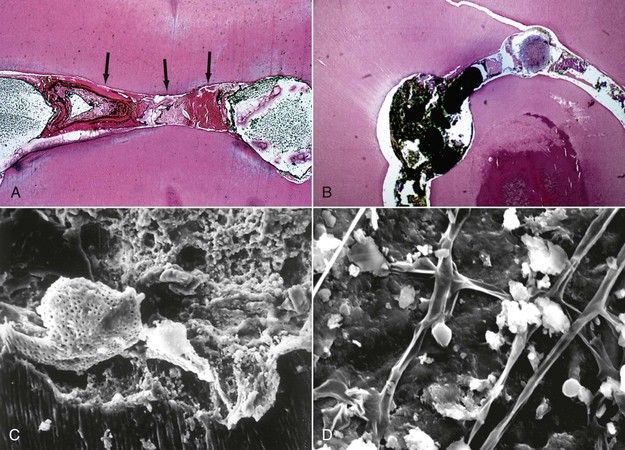
FIGURE 11-1 A, Tissue debris sandwiched in the anastomoses between the mesial buccal and mesial lingual canals of the mandibular molar after cleaning and obturation. B, Tissue debris that remains in a C-Shaped canal system after cleaning and shaping (H&E stain ×10). C, Scanning electron micrograph (SEM) shows the smear layer that is present during the shaping of the root canal is packed with tissue debris, dentin chips, and bacteria (×750). D, SEM showing surface tissue debris in anatomic irregularities after shaping and cleaning the root canal with irrigants (×2000).
No one solution as yet possesses all the properties of an ideal irrigant, but it is important to emphasize that the use of neutral solutions for the irrigation process (e.g., water, saline, anesthetic solutions) serves no useful purpose in the root canal system.

CLINICAL PROBLEM
Solution
The best irrigant is sodium hypochlorite (NaOCl) in various concentrations. NaOCl is a highly effective irrigating solution that is both antimicrobial and has tissue-dissolving capabilities.

The effectiveness of NaOCl in cleaning and disinfection depends on the concentration of available chlorine and the pH of the solution. Hypochlorous acid (HOCl) is a weak acid and dissociates to the hypochlorite ion (OCl−) and proton (H+) depending on the solution pH. It is generally believed that HOCl is the active species in the germicidal action, whereas the concentration of OCl− is a key factor determining cleaning efficiency. This implies that the optimal pH region of the germicidal activity of NaOCl differs from that of its cleaning activity.
The concentrations of NaOCl that are used during root canal procedures vary among clinicians and may very well be chosen empirically. Some bacterial populations are susceptible to percentages as low as 0.5%,
Using the NaOCl solutions for periods of 5 to 30 minutes in the root canal has also been advocated to enhance its effectiveness, but optimal times have not been determined.
NaOCl should always be delivered passively to the canal to prevent a forceful extrusion beyond the apical foramen. This delivery is best accomplished by avoiding wedging the delivery needle in the canal, expressing the solution slowly, and using specially tipped side-delivery needles that enhance the cleaning of the dentin walls while minimizing potential risks during its use (
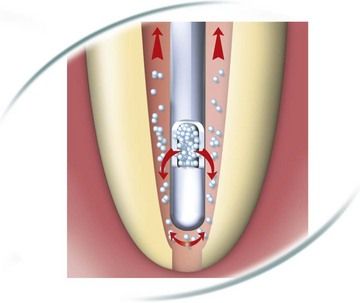
FIGURE 11-2 Side-delivery needle enables delivery of irrigating solutions to the root canal walls to enhance canal cleaning.
(Courtesy Dentsply Maillefer, Ballaigues, Switzerland.)
Claims have also been made relative to the application of ultrasonic irrigation devices alone and during canal cleaning and shaping.
A development that has claimed to eliminate problems with the delivery of NaOCl is the EndoVAC (Discus Dental, Culver City, CA, USA). The device offers an apical negative pressure system that uses microcannulas to deliver irrigants to the canal and draws fluid away by evacuation. Claims are made that cleaning occurs to within 1 mm of the apex,
The most recent addition to this foray of irrigation devices is the ProUltra PiezoFlow irrigation device (Dentsply Tulsa Dental Specialties, Tulsa, OK, USA). ProUltra PiezoFlow Ultrasonic Irrigation Needles are used to deliver irrigants by application of ultrasonic vibration. The PiezoFlow irrigation needles are used in conjunction with a piezo-electric ultrasonic energy-generating unit to provide the energy for tip oscillation. A syringe or other irrigation source is attached to the Luer-lock connection on the ultrasonic needle. Removal of irrigant is through the operatory vacuum source. Compatible irrigants include sodium hypochlorite (up to 6%), EDTA (up to 17%), Chlorhexidine (up to 2%) and BioPure MTAD, however working times with each irrigant may vary.
NaOCl contact with dentin has been questioned as to its impact on the mechanical, chemical, and structural composition of dentin.
NaOCl should not be used as the final rinsing agent when resin-based bonded root canal filling materials are planned for canal obturation. The bonding of the sealer to the dentin may be altered. Alternatives are to finish with EDTA, chlorhexidine (CHX),
NaOCl has strong support as being the best intracanal irrigant
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


