Chapter 10 The tooth-coloured restorative materials IV
Resin-modified glass ionomer cements
Learning Objectives
From this chapter, the reader will:
• Understand what resin-modified glass ionomers are
• Appreciate the significance of their constituents and their influence on the properties of the material
• Understand how the constituents impact on the material’s clinical performance
• Be aware of the applications and be aware of currently available commercial products
• Have an appreciation of how to use these family of materials more effectively
Introduction and History of Development
Resin-modified glass ionomer cements (RMGICs) were developed in an attempt to address the perceived limitations of the conventional glass ionomer cements and to utilize the advantages of resin composite materials (Table 10.1). In other words, RMGICs aim to maintain the benefits of fluoride ion release and adhesion of glass ionomer cements while overcoming their disadvantages with the more favourable attributes of resin-based composites.
Table 10.1 Advantages of resin-based composites and disadvantages of glass ionomer cements in relation to the development of resin-modified glass ionomers
| Advantages of resin-based composites | Disadvantages of glass ionomer cements |
|---|---|
| Command set by visible light | Low early strength |
| Better wear resistance | Susceptibility to erosion |
| Lower solubility | Slow setting phase |
| Early high compressive and flexural strength | Risk of desiccation |
| Good polishability | |
| Ability to retain its polish |
Components of A Resin-Modified Glass Ionomer
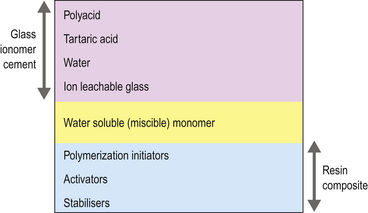
Essentially RMGICs are conventional glass ionomer cements containing glass, polyacid, tartaric acid and water, with the addition of a water-soluble resin and modified poly(acrylic acids) (Figure 10.1). These modified polyacrylic acids have pendant methacrylate groups or methacryloxy groups grafted onto the polyacid chain and are called copolymers. Chemicals that allow light activation are also incorporated. These materials may therefore be regarded as hybrid glass ionomers. They behave primarily as glass ionomer cements as only a small amount of resin has been added, which is usually hydroxyethylmethacrylate (HEMA).
What is HEMA?
Biocompatibility of HEMA
The HEMA monomer is cytotoxic and so care should be taken in the application of the unset material to any soft tissue. The cytotoxicity is minimal after polymerization, however, if the level of conversion is low then unconverted monomer can leach out as polyHEMA, which will absorb water very quickly. HEMA is known to cause chemical dermatitis (Figure 10.2) if it comes into contact with skin or mucous membrane.
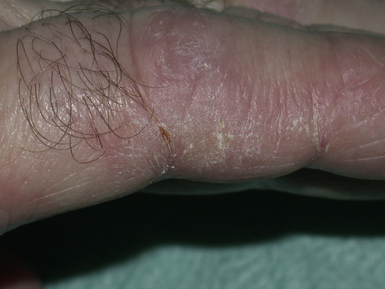
Fig. 10.2 Chemical dermatitis in an active state. Note the cracks on the skin and the erythema (redness).
Furthermore if the polymer degrades, the resulting release of freed monomer into the surrounding dental hard and surrounding tissues may potentially have toxicological effects on the dental pulp and osteoblasts (see Chapter 3).
Even in its polymerized form bound in the RMGIC, if HEMA is placed directly onto vital dental pulpal tissue it may cause the death of the pulpal tissue. For this reason the use of RMGICs in direct contact with the pulp is contraindicated. In a very deep cavity, micro-exposures may be present and not obvious to the dentist. If there is any doubt, consider the use of another lining material such as a setting calcium hydroxide cement placed on the pulpal floor as a sublining. A layer of RMGIC may then be placed over the set calcium hydroxide cement and the surrounding dentine. This will seal the dentinal tubules opened during cavity preparation and decrease the risk of microleakage (Figure 10.3).
Chemical constituents of RMGICs
The chemical constituents of RMGICs are shown in Table 10.2.
Table 10.2 Chemical components of a resin-modified glass ionomer cement
| Powder | Liquid | Purpose for their inclusion |
|---|---|---|
| Barium, strontium or aluminosilicate glass | Improved strengthImparts radiopacity | |
| Vacuum-dried polyacrylic acid | Polyacrylic acid | Reacts with the glass to form the poly salt matrix |
| Potassium persulphate | Redox catalyst system to provide the methacrylate (dark) cure | |
| Ascorbic acid | ||
| Pigments | Varies shade | |
| HEMA | Water miscible resin | |
| Polyacrylic acid with pendant methacrylates (copolymer) | Ability to undergo both acid–base and polymerization reactions Helps form interpenetrating network | |
| Tartaric acid | Sharpens the acid–base reaction set | |
| Water | Permits reaction between the polyacid and the glass | |
| Photo-initiators | Achieves light curing |
Setting Reaction
The polyacrylic acid reacts with the glass to form the poly salt matrix as for the conventional glass ionomer cement (see Chapter 9). This extended setting reaction means that the salt matrix is liable to damage for some time after placement if exposed to water. The addition of a water-miscible monomer to the material permits a polymerization reaction to take place during the initial stages of the setting of the material, which provides it with early strength as the secondary acid–base reaction between the glass and the polyacrylic acid goes to completion. In other words, the polymerized resin phase is designed to form a scaffolding while the ionomer cement matrix is being formed. As the resin content is increased so the acid–base reaction is slowed. The effect of the resin addition is primarily seen during the initial stages of set when the glass ionomer cement is at its weakest. It is essential that the resin is soluble in water as the cement remains water based. In the absence of water, no reaction will occur between the polyacid and the glass. This restriction limits the number of resin systems which may be used in these materials as most are hydrophobic.
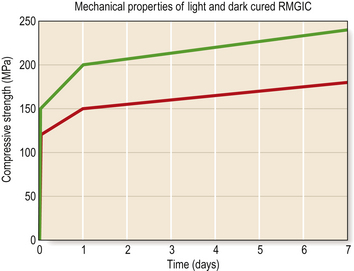
Reliance on the redox reaction for the cure leads to a reduction in the level of conversion of the material and reduced mechanical properties. This is illustrated in Figure 10.4, which shows the difference in compressive strength of a RMGIC when light cured and when the redox setting reaction is relied on alone. The magnitude of shrinkage is in excess of the resin-based composites so considerable care must be taken to control the effect of the stresses which are generated. In thin sections, particularly when used as a liner, the margins of the cement layer may curl up away from the surface to be protected. As with the conventional acid–base cements, the acid–base reaction is not involved with the shrinkage. It will only contribute if there is desiccation of the material.
Dual- and tri-cure
The two types of setting reaction seen in RMGICs are referred to as:
Stages of the RMGIC setting reaction
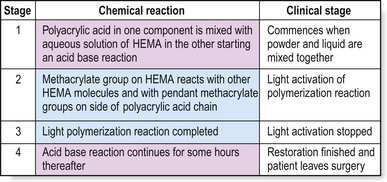
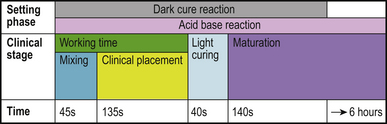
The setting reaction of RMGICs is complex as two or three chemical reactions are occurring concurrently depending on whether the product is a dual- or tri-cure material (Figure 10.5).
Dual-cured products
• Acid–base reaction: This commences at the start of mixing and often continues for a substantial time after all other setting reactions have been completed, which may be up to 6 hours from start of mixing. During this time, the matrix is susceptible to damage by extraneous water.
• Light activation: This takes place at the end of placement and is completed within 10 seconds of light activation. Little post curing occurs but the material in the path of the light will have formed a solid resin matrix at this point. Water uptake by the polymer will start from the saliva in the oral cavity at this time.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses