1
Implant Abutment Materials
1Washington Hospital Center, Department of Oral and Maxillofacial Surgery, Washington, DC; and American Institute of Implant Dentistry, Washington, DC
2Private Practice, Gilbert, AZ
Introduction
A wide variety of abutment materials are available on the dental implant market. A major challenge for clinicians today is understanding the biologic response to each material, as well as the best indication for using each of the different types.
To complicate this problem, there are no well defined and comprehensive sources reviewing the properties associated with abutment materials. This chapter provides relevant information on abutment materials and their soft tissue response.
Mucosal Seal
The mucosal seal surrounding a dental implant abutment is an essential factor in preventing bacterial penetration into the crestal bone and around the implant neck. In order to understand the soft tissue response, it is important to be familiar with the anatomy of the mucosal seal.
Natural Dentition
The periodontal soft tissue is an important factor in a person’s natural protection against periodontal disease. The biologic width is the depth of soft tissue below the sulcus in the natural dentition. It consists of a junctional epithelium and connective tissue layer. The junctional epithelium ranges from 1 to 2 mm wide followed apically by a 1 mm layer of connective tissue. The alveolar bone lies just below this connective tissue.
In the natural dentition, this zone has been proven to be essential for protecting the periodontium from plaque and bacteria penetration into the oral cavity. The junctional epithelium attaches to the teeth with a hemidesmisomal attachment, providing a shield against bacteria. The connective tissue layer contains collagen fibers that insert into the teeth and cementum perpendicularly to the tooth. These fibers provide additional reinforcement against an apically migrating junctional epithelium caused by periodontal disease.
Peri-implant Mucosal Seal
A mucosal seal surrounding dental implants is also essential in avoiding peri-implantitis. The biologic width surrounding dental implants also contains a junctional epithelium, followed apically by a connective tissue layer. As in the natural dentition, the coronal portion of the biologic width contains the junctional epithelium. In 1984, Gould and colleagues demonstrated that this junctional epithelium attaches to the titanium surface in a similar manner to the natural dentition, with hemidesmosomes. A connective tissue attachment can be found further apically. Buser et al. (1992) described this attachment as being rich in collagen fibers but sparse in cells or resembling scar tissue.
Unlike the natural dentition, in implant abutments the apical connective tissue fibers do not have the same quality of attachments. The natural dentition has dentogingival fibers running perpendicular to the tooth from the bone to the cementum. The connective tissue layer surrounding a dental implant abutment has fibers running in a parallel fashion (Figure 1.1). The only exception to this histology is with Laser-Lok™ abutments which are discussed later in this chapter.
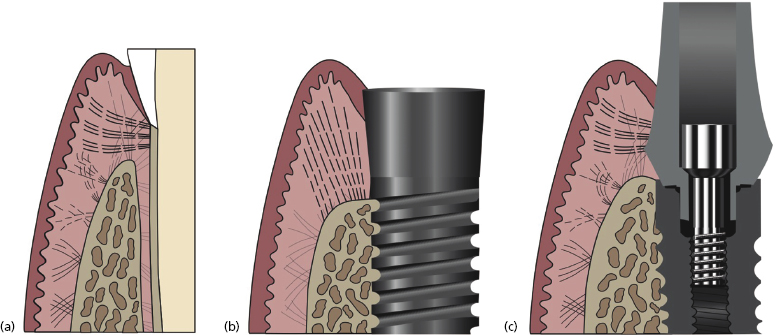
Due to the weakened connective tissue support around implant abutments, the junctional epithelium is believed to be more susceptible to apical migration. In other words, a dental implant is more susceptible to peri-implantitis than a natural tooth is to periodontitis.
It is important to note that this biologic width or “peri-implant seal” protects the implant against peri-implantitis and provides an esthetic result. When considering which abutment type to use one should consider how well the abutment forms and maintains this mucosal seal.
Pellicle, Biofilm, and Periodontal Disease
One of the key factors in selecting an abutment material is its hygienic property. To review the importance of hygiene it is important to understand pellicle formation, subsequent biofilm production, and the pathway of peri-implantitis development.
Pellicle
The process of plaque formation begins with glycoproteins attaching to the surface of the enamel or an abutment, creating a thin layer called the pellicle. Although this layer by itself is harmless, it provides a framework for bacteria to adhere to.
Biofilm
A biofilm is an aggregation of multiple organisms coexisting together. Initially, Gram-positive aerobic cocci adhere to this thin glycoprotein layer or pellicle. As these bacteria multiply, the bacterial colonies multiply creating a more anaerobic environment. This anaerobic environment then permits more harmful Gram-negative rods to collect within the biofilm. The biofilm creates an acidic environment that contributes to dental caries but, more relevant to the topic at hand, the biofilm also contributes to periodontal disease.
Periodontal Disease in the Natural Dentition
Periodontal disease is caused by the biofilm, which destroys the periodontium and causes loss of the alveolar bone and inflammation of the periodontal tissues. This is not a novel development – the landmark paper by Page and Schroeder outlined this process of periodontal disease back in 1976.
Peri-implantitis
As in the natural dentition, development of the pellicle and biofilm and subsequent inflammation also occurs with dental implants. This process can cause the potential for apical migration of the peri-implant seal and bone loss. The process of peri-implantitis is more common with dental implants than periodontal disease is with natural dentition. This is because the peri-implant mucosal seal is not as effective (except in the case of Laser-Lok abutments) as the mucosal seal surrounding the natural dentition.
As will be discussed, some abutments have enhanced capabilities for resisting bacterial colonization. Other abutments have improved capabilities for forming a more resistant mucosal seal with a strengthened connective tissue attachment.
Implant Abutment Material Related Research
The remainder of this chapter focuses on the variety of abutments available on the market. Different abutment materials will be compared in terms of their ability to form and maintain the “peri-implant seal.” Carefully chosen research has been selected to demonstrate how the varieties of abutments specifically affect soft tissue.
The most commonly used implant abutment materials (Figure 1.2, Table 1.1) to be discussed are:
- Titanium:
- machined
- polished
- Laser-Lok.
- Surgical grade stainless steel.
- Cast gold.
- Zirconia.
- Polyether ether ketone (PEEK).

Table 1.1 Abutment materials and soft tissue response
| Abutment material | Forming the peri-implant seal | Maintaining the peri-implant seal |
|---|---|---|
| Titanium (machined or polished) | Long-term studies supporting favorable soft tissue results with machined or polished titanium. Most validated abutment material in the literature | Long-term studies supporting favorable soft tissue maintenance with machined or polished titanium. Most validated abutment material in the literature |
| Titanium abutments with a Laser-Lok transmucosal collar | Greatest ability to form a connective tissue attachment compared with all other abutment materials on the market | Strongest peri-implant seal permitting improved long-term soft tissue maintenance (comparable mucosal seal to the natural dentition) |
| Gold | Conflicting studies in the literature concerning the ability to form an adequate peri-implant seal | Conflicting studies concerning the long-term maintenance of the peri-implant seal |
| PEEK (polyether ether ketone) | Comparable soft tissue results to titanium | Comparable hygienic properties to titanium |
| Zirconia | Comparable ability to form a peri-implant seal to that of machined or polished titanium | Most hygienic abutment on the market allowing improved long-term maintenance of the peri-implant seal |
Titanium
Physical properties
Titanium is the only element that offers the unique combination of strength, light weight, and biocompatibility, as well as being extremely durable and strong. Titanium has high corrosion resistant and the highest strength to weight ratio of any known element (Figure 1.3).
Titanium abutments are either made of commercially pure titanium or titanium alloy.
Commercially pure titanium
Commercially pure (CP) titanium is widely utilized for medical applications because of its corrosion resistant, high strength, and biocompatible applications. The mechanical properties of CP titanium are influenced by small additions of oxygen and iron. By careful control of these additions, the various grades of CP titanium are produced to give properties suited to different applications. CP titanium with the lowest oxygen and iron levels makes the most formable grade of material; while progressively higher oxygen content results in higher strength levels.
Color
Titanium abutments come either with a silver gold color coating (Figure 1.4).
The gold color coating over the surface of the abutment is called titanium nitride. The titanium nitride (TiN; sometimes known as “Tinite,” “TiNite,” or “TiN”) coating is created by a plasma coating process in which titanium and nitrogen ions are combined with TiN, and then molecularly bonded with the titanium substrate of the abutment. TiN was first used in the medical device industry in the 1980s. Biocompatibility testing has been conducted on TiN over many years and this testing, as well as subsequent clinical applications, has demonstrated that TiN is biocompatible and appropriate for use in implantable medical devices that come in contact with bone, skin, tissues, or blood (Figure 1.5).
Titanium nitride is an extremely hard ceramic material, often used as a coating over the titanium component to not only improve the substrate’s surface properties but also to achieve a warm, esthetic tone under the gingiva because of its gold shaded hue. Generally, the TiN coating covers the entire abutment except for the contact area between the abutment/implant and screw/abutment. This type of titanium abutment is ideal for esthetically challenging cases with thin soft tissue or when using an all-ceramic crown. In most applications the TiN coating is less than 5 micrometers (0.00020 inches) thick. This coating is only meaningful with CAD/CAM milled abutments where the abutment is not adjusted. Prefabricated abutments are adjusted and generally will lose any strength added by the nitrates following the abutment adjustment.
Titanium alloy (Ti-6Al-4V, Ti6Al4V, or Ti-6-4)
Titanium alloy is also called grade 5 titanium. Titanium alloy contains 6% aluminum, 4% vanadium, 0.25% (maximum) iron, 0.2% (maximum) oxygen, and the remainder titanium. Ti-6Al-4V alloy is significantly stronger than commercially pure titanium and offers better tensile strength and fracture resistance (Figure 1.6).

Because of titanium’s unique physical properties, titanium abutments are the first choice for posterior implants. These abutments are available as prefabricated stock or CAD/CAM milled customized abutments.
There is an extensive literature validating the favorable soft tissue response with titanium abutments. Because the majority of the research about peri-implant tissue and abutment materials is based on titanium abutment, this material has become a reference point in describing the properties of other abutment materials.
Machined versus polished titanium and soft tissue responses
Surface roughness is the key difference between machined and polished titanium. This section evaluates whether there is a clinically significant difference between the soft tissue response to polished and machined titanium.
The break down of the peri-implant seal is brought on by the development of a pellicle, biofilm, and inflammation followed by alveolar bone loss. It is well established that the initial glycoproteins and biofilm are more likely to attach to a rough surface than a smooth one. With this logic it could be wrongly assumed that abutments with a smoother surface have less inflammatory response, thus less bone resorption. However, multiple clinical studies have failed to show a clinically significant relationship between an inflammatory response and a roughened abutment surface.
To provide one of many examples, Zitzmann’s study concluded that there was no relation between inflammatory response and the abutment surface roughness (Abrahamsson et al. 2002).
In conclusion, although it has been shown that bacteria are more likely to aggregate on a roughened surface, clinical studies between titanium abutments on the market fail to show this relationship. There is no clinically significant different soft tissue response to machined and polished titanium.
Prefabricated abutments with a Laser-Lok surface characteristic are a new innovative product (Figure 1.7). The Laser-Lok consists of 8–12 micron titanium micro-channels. These micro-channels provide the following advantages:
- They enhance the establishment of a connective tissue attachment.
- They inhibit the apical migration of the junctional epithelium.
- They preserve the crestal bone.

With all other implant abutments on the market, connective tissue forms in a weakened parallel fashion to the abutment. The Laser-Lok technology enables the formation of />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


