Chapter 1 Dental materials in the oral environment
Learning Objectives
From this chapter, the reader will:
• Appreciate the environment within which dental materials are designed to function
• Understand the common presentations of dental materials
• Appreciate the pitfalls of the manipulation of dental materials in the clinic by the dental team and the significance of these on material performance
• Understand how the presentation of materials has evolved to reduce such operator variability.
Introduction
Perhaps surprisingly, when considering their diversity, the materials that are used across the various branches of dentistry have much in common. They are conceived, developed, tested, manufactured and marketed in the same way by only a small group of dental material manufacturing companies. When they arrive in the clinic, they are kept in the same storeroom. When required for use, they are mixed and manipulated by the dental team in similar ways as they are available in only a small number of presentations. Unfortunately, incorrect handling during this phase could compromise their properties on subsequent placement into the mouth and in some cases they may be ruined. Dental materials may set chemically, or the setting may be initiated by light energy, or a combination of both. They are subjected to the hostile environment which is the human mouth, where they are asked to endure similar stresses and strains during function. With all things being equal their lifespan does not vary significantly either. The first section of this book, consisting of five chapters, discusses these general principles and the commonalities between the different material groups.
This chapter describes the oral environment into which a material is placed. Failure of the clinician to understand the conditions in which a material is expected to perform may lead to incorrect material selection – which will surely compromise clinical success. The way in which the material is presented and handled by chairside dental staff will influence its properties. In order to minimize operator error, manufacturers have developed less technique-sensitive presentations. Chapter 2 examines how the dental team may influence success by considering clinical factors such as the importance of moisture control when using materials which may be damaged by exposure to water prior to their complete setting. The invention and extensive use in modern dentistry of setting dental materials by exposure to light energy extends across material types. However, this useful technique has the potential to be compromised by various factors and the dentist must be aware of the potential pitfalls.
It is an important criterion that it is undesirable for any dental material to interact with the host, i.e. dental patient. Chapters 3 and 4 deal with the biological considerations of dental materials and how potential interactions may be minimized by the manufacturers and dental team. Clearly all materials must be fit for purpose and conform to various safety, legal and quality standards.
Prior to their launch to market, all dental materials are tested extensively. It is desirable that the end user (the dentist) understands which laboratory tests each material has been subjected to and how relevant this may be clinically. Often promotional and technical literature may be difficult to interpret, yet it is vital that the dentist is equipped to understand this information. By understanding why certain chemicals are contained in the materials it may be possible to make an educated assessment as to how the material is likely to behave with respect to handling and function. This will enable the clinician to make informed decisions as to whether a particular material is suitable for a given situation. Lastly, Chapter 5 describes how materials may be stored and managed in a clinic or dental practice prior to use. Storage in the correct manner will ensure that the material reaches the dentist in optimum condition.
The Hostile Oral Environment
When dental materials are placed in the mouth, they enter a very hostile environment. In fact, they are asked to perform and survive in conditions more extreme than those found on the oil platforms in the North Sea. There are numerous microorganisms in the oral cavity, and many bacteria produce acids as a by-product of their metabolism. This has the effect of lowering the intraoral pH. Ingested foodstuffs and liquids can also rapidly change the pH of the mouth, swinging between mildly alkaline and strongly acid. Saliva plays a role as a protective fluid barrier by acting as a buffer. However, some of the constituents of saliva such as acids and organic fluids can cause degradation of the dental restoration with time by reacting with it chemically.
pH: stands for the power of the Hydrogen ion and is a measure of the acidity or basicity of a solution. It ranges from 0.1 (highly acid) to 14 (highly alkaline), with pH 7 being neutral.
Buffer solution: an aqueous solution in which the change in pH of the solution is limited when a small amount of acid or base is added to it. Buffer solutions are used as a means of maintaining pH at a nearly constant value.
(Dental) restoration: a form composed of a dental material designed to restore both form and function of dental hard tissue.
Stress: measure of the intensity or internal distribution of the total internal forces acting within a deformable body. Stress is measured in N/m2 (newtons/metre2).
Strain: the change in the dimension of a material per unit length when an external force is applied to it.
The influence of the presentation of materials on success
Powder and liquid presentations
Powder versus granules
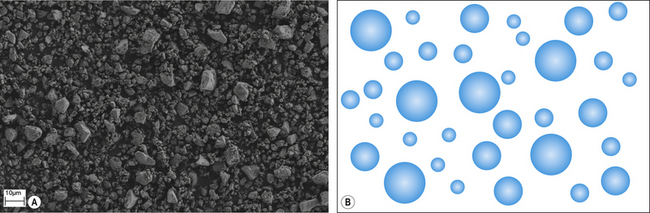
The ease with which the solute dissolves affects the rate of reaction. Generally, speaking dental cements are presented in either powder, or in the case of newer materials, granular form. A powder (Figure 1.2) is a fine precipitate produced by grinding or milling small particles. The finer the particle the more rapid the initiation of the chemical reaction as the surface area to volume ratio increases. Granules are larger agglomerates of semi-fused particles with a porous structure (Figure 1.3), which facilitates the permeability of a liquid into the agglomerated mass and the surface area available is greater for reaction. This is a similar presentation to the popular coffee granules found in some instant coffees. Figure 1.4 shows the granular and powered presentations of instant coffee. The granular variety was introduced to improve the rapidity with which the coffee may dissolve when hot water is added to it.
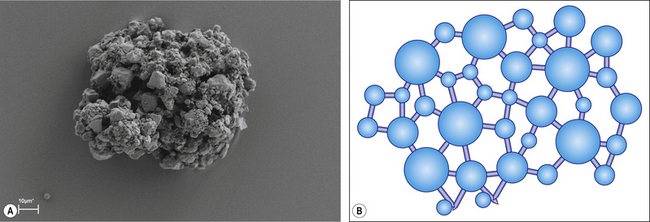
Fig. 1.3 A scanning electron micrograph and pictorial representation of a granular glass ionomer powder.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses