Anatomy of the Periodontium
The normal periodontium provides the support necessary to maintain teeth in function. It consists of four principal components: gingiva, periodontal ligament, cementum, and alveolar bone. Each of these periodontal components is distinct in its location, tissue architecture, biochemical composition, and chemical composition, but all of these components function together as a single unit. Recent research has revealed that the extracellular matrix components of one periodontal compartment can influence the cellular activities of adjacent structures. Therefore, the pathologic changes that occur in one periodontal component may have significant ramifications for the maintenance, repair, or regeneration of other components of the periodontium.18
Oral Mucosa
The oral mucosa consists of the following three zones:
1. The gingiva and the covering of the hard palate, termed the masticatory mucosa (The gingiva is the part of the oral mucosa that covers the alveolar processes of the jaws and surrounds the necks of the teeth.)
2. The dorsum of the tongue, covered by specialized mucosa
3. The oral mucous membrane lining the remainder of the oral cavity
Gingiva
Clinical Features
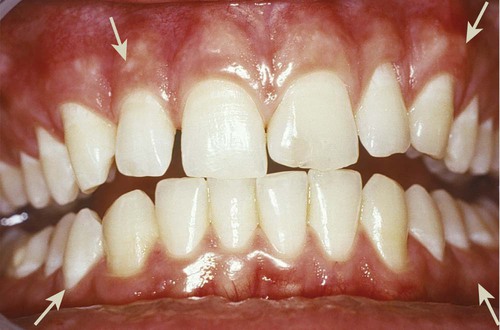
In an adult, normal gingiva covers the alveolar bone and tooth root to a level just coronal to the cementoenamel junction. The gingiva is divided anatomically into marginal, attached, and interdental areas. Although each type of gingiva exhibits considerable variation in differentiation, histology, and thickness according to its functional demands, all types are specifically structured to function appropriately against mechanical and microbial damage.7 In other words, the specific structure of different types of gingiva reflects each one’s effectiveness as a barrier to the penetration by microbes and noxious agents into the deeper tissue.
Marginal Gingiva.
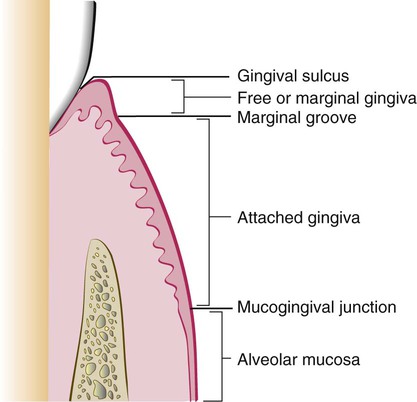
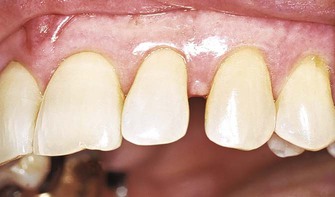
The marginal or unattached gingiva is the terminal edge or border of the gingiva that surrounds the teeth in collarlike fashion (Figures 1-1 and 1-2).6 In about 50% of cases, it is demarcated from the adjacent attached gingiva by a shallow linear depression called the free gingival groove.6 The marginal gingiva is usually about 1 mm wide, and it forms the soft-tissue wall of the gingival sulcus. It may be separated from the tooth surface with a periodontal probe. The most apical point of the marginal gingival scallop is called the gingival zenith. Its apicocoronal and mesiodistal dimensions vary between 0.06 and 0.96 mm.171
Gingival Sulcus.
The gingival sulcus is the shallow crevice or space around the tooth bounded by the surface of the tooth on one side and the epithelium lining the free margin of the gingiva on the other side. It is V-shaped, and it barely permits the entrance of a periodontal probe. The clinical determination of the depth of the gingival sulcus is an important diagnostic parameter. Under absolutely normal or ideal conditions, the depth of the gingival sulcus is 0 mm or close to 0 mm.105 These strict conditions of normalcy can be produced experimentally only in germ-free animals or after intense and prolonged plaque control.13,49
In clinically healthy human gingiva, a sulcus of some depth can be found. The depth of this sulcus, as determined in histologic sections, has been reported as 1.8 mm, with variations from 0 to 6 mm195; other studies have reported 1.5 mm289 and 0.69 mm.93 The clinical evaluation used to determine the depth of the sulcus involves the introduction of a metallic instrument (i.e., the periodontal probe) and the estimation of the distance it penetrates (i.e., the probing depth). The histologic depth of a sulcus does not need to be exactly equal to the depth of penetration of the probe. The penetration of the probe depends on several factors, such as probe diameter, probing force, and level of inflammation.91 Consequently, the probing depth is not necessarily exactly equal to the histologic depth of the sulcus. The so-called probing depth of a clinically normal gingival sulcus in humans is 2 to 3 mm (see Chapter 30).
Attached Gingiva.
The attached gingiva is continuous with the marginal gingiva. It is firm, resilient, and tightly bound to the underlying periosteum of alveolar bone. The facial aspect of the attached gingiva extends to the relatively loose and movable alveolar mucosa; it is demarcated by the mucogingival junction (see Figure 1-2).
The width of the attached gingiva is another important clinical parameter.7 It is the distance between the mucogingival junction and the projection on the external surface of the bottom of the gingival sulcus or the periodontal pocket. It should not be confused with the width of the keratinized gingiva, although this also includes the marginal gingiva (see Figure 1-2).
The width of the attached gingiva on the facial aspect differs in different areas of the mouth.40 It is generally greatest in the incisor region (i.e., 3.5 to 4.5 mm in the maxilla, 3.3 to 3.9 mm in the mandible) and narrower in the posterior segments (i.e., 1.9 mm in the maxillary first premolars and 1.8 mm in the mandibular first premolars)6 (Figure 1-3).
Because the mucogingival junction remains stationary throughout adult life,4 changes in the width of the attached gingiva are caused by modifications in the position of its coronal portion. The width of the attached gingiva increases by the age of 4 years and in supraerupted teeth.5 On the lingual aspect of the mandible, the attached gingiva terminates at the junction of the lingual alveolar mucosa, which is continuous with the mucous membrane that lines the floor of the mouth. The palatal surface of the attached gingiva in the maxilla blends imperceptibly with the equally firm and resilient palatal mucosa.
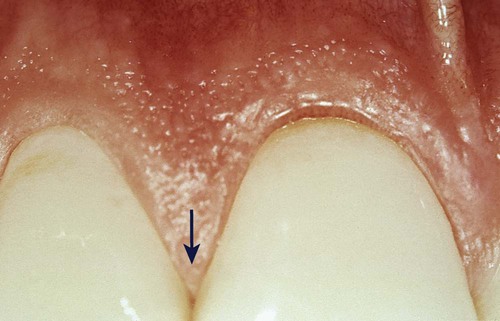
Interdental Gingiva.
The interdental gingiva occupies the gingival embrasure, which is the interproximal space beneath the area of tooth contact. The interdental gingiva can be pyramidal, or it can have a “col” shape. In the former, the tip of one papilla is located immediately beneath the contact point; the latter presents a valleylike depression that connects a facial and lingual papilla and that conforms to the shape of the interproximal contact62 (Figures 1-4 and 1-5). The shape of the gingiva in a given interdental space depends on the presence or absence of a contact point between the adjacent teeth, the distance between the contact point and the osseous crest,260 and the presence or absence of some degree of recession. Figure 1-6 depicts the variations in normal interdental gingiva.
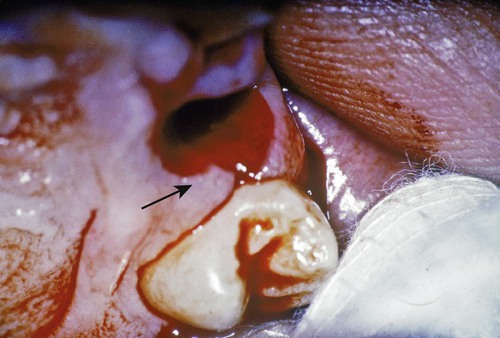
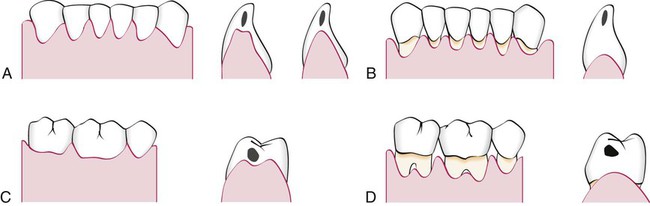
The facial and lingual surfaces are tapered toward the interproximal contact area, whereas the mesial and distal surfaces are slightly concave. The lateral borders and tips of the interdental papillae are formed by the marginal gingiva of the adjoining teeth. The intervening portion consists of attached gingiva (Figure 1-7). If a diastema is present, the gingiva is firmly bound over the interdental bone to form a smooth, rounded surface without interdental papillae (Figure 1-8).
Microscopic Features
Microscopic examination reveals that gingiva is composed of the overlying stratified squamous epithelium and the underlying central core of connective tissue. Although the epithelium is predominantly cellular in nature, the connective tissue is less cellular and composed primarily of collagen fibers and ground substance. These two tissues are considered separately.*
Gingival Epithelium
General Aspects of Gingival Epithelium Biology.
Historically, the epithelial compartment was thought to provide only a physical barrier to infection and the underlying gingival attachment. However, we now believe that epithelial cells play an active role in innate host defense by responding to bacteria in an interactive manner,67 which means that the epithelium participates actively in responding to infection, in signaling further host reactions, and in integrating innate and acquired immune responses. For example, epithelial cells may respond to bacteria by increased proliferation, the alteration of cell-signaling events, changes in differentiation and cell death, and, ultimately, the alteration of tissue homeostasis.67 To understand this new perspective of the epithelial innate defense responses and the role of epithelium in gingival health and disease, it is important to understand its basic structure and function (Box 1-1).
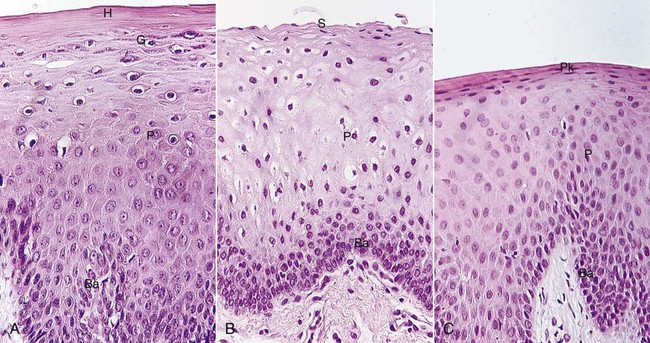
Differentiation involves the process of keratinization, which consists of progressions of biochemical and morphologic events that occur in the cell as they migrate from the basal layer (Figure 1-9). The main morphologic changes include the following: (1) the progressive flattening of the cell with an increasing prevalence of tonofilaments; (2) the couple of intercellular junctions with the production of keratohyalin granules; and (3) the disappearance of the nucleus. (See Schroeder230 for further details.)
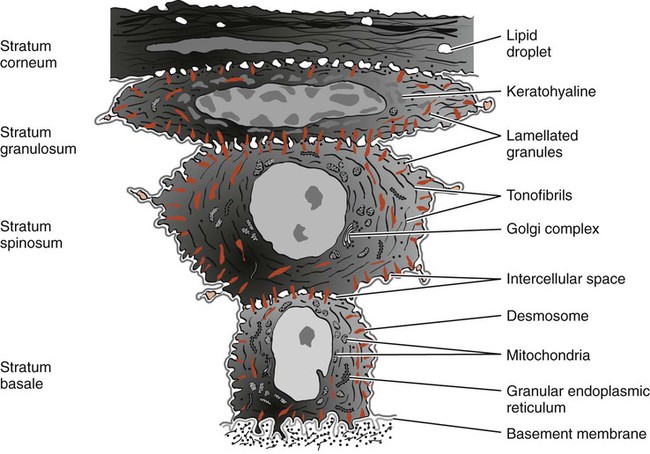
A complete keratinization process leads to the production of an orthokeratinized superficial horny layer similar to that of the skin, with no nuclei in the stratum corneum and a well-defined stratum granulosum (Figure 1-10). Only some areas of the outer gingival epithelium are orthokeratinized; the other gingival areas are covered by parakeratinized or nonkeratinized epithelium45 and considered to be at intermediate stages of keratinization. These areas can progress to maturity or dedifferentiate under different physiologic or pathologic conditions.
Immunohistochemistry, gel electrophoresis, and immunoblot techniques have made the identification of the characteristic pattern of cytokeratins possible in each epithelial type. The keratin proteins are composed of different polypeptide subunits characterized by their isoelectric points and molecular weights. They are numbered in a sequence that is contrary to their molecular weight. In general, basal cells begin synthesizing lower-molecular-weight keratins (e.g., K19 [40 kD]), and they express other higher-molecular-weight keratins as they migrate to the surface. K1 keratin polypeptide (68 kD) is the main component of the stratum corneum.60
Thus, in the fully differentiated state, the corneocytes are mainly formed by bundles of keratin tonofilaments embedded in an amorphous matrix of filaggrin and surrounded by a resistant envelope under the cell membrane. The immunohistochemical patterns of the different keratin types, envelope proteins, and filaggrin change under normal or pathologic stimuli, thereby modifying the keratinization process.128–130
Electron microscopy reveals that keratinocytes are interconnected by structures on the cell periphery called desmosomes.154 These desmosomes have a typical structure that consists of two dense attachment plaques into which tonofibrils insert and an intermediate, electron-dense line in the extracellular compartment. Tonofilaments, which are the morphologic expression of the cytoskeleton of keratin proteins, radiate in brushlike fashion from the attachment plaques into the cytoplasm of the cells. The space between the cells shows cytoplasmic projections that resemble microvilli and that extend into the intercellular space and often interdigitate.
Less frequently observed forms of epithelial cell connections are tight junctions (zonae occludens), in which the membranes of the adjoining cells are thought to be fused.268,287 Evidence suggests that these structures allow ions and small molecules to pass from one cell to another.
Conversely, enzymes of the pentose shunt (an alternative pathway of glycolysis), such as glucose-6-phosphatase, increase their activity toward the surface. This pathway produces a larger amount of intermediate products for the production of ribonucleic acid (RNA), which in turn can be used for the synthesis of keratinization proteins. This histochemical pattern is in accordance with the increased volume and the amount of tonofilaments observed in cells reaching the surface; the intensity of the activity is proportional to the degree of differentiation.72,82,127,202
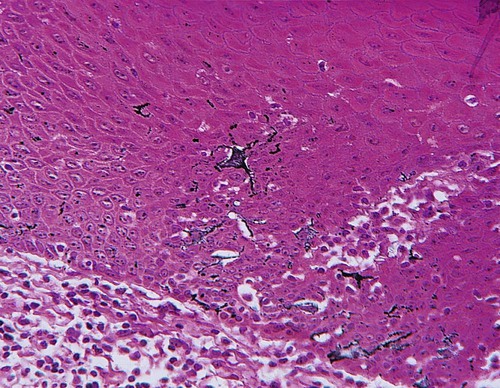
The uppermost cells of the stratum spinosum contain numerous dense granules called keratinosomes or Odland bodies, which are modified lysosomes. They contain a large amount of acid phosphatase, an enzyme involved in the destruction of organelle membranes, which occurs suddenly between the granulosum and corneum strata and during the intercellular cementation of cornified cells. Thus, acid phosphatase is another enzyme that is closely related to the degree of keratinization.46,125,284 These contain tyrosinase, which hydroxylates tyrosine to dihydroxyphenylalanine (dopa), which in turn is progressively converted to melanin. Melanin granules are phagocytosed and contained within other cells of the epithelium and connective tissue called melanophages or melanophores.
Non-keratinocyte cells are present in gingival epithelium as in other malpighian epithelia. Melanocytes are dendritic cells located in the basal and spinous layers of the gingival epithelium. They synthesize melanin in organelles called premelanosomes or melanosomes61,228,252 (Figure 1-11).
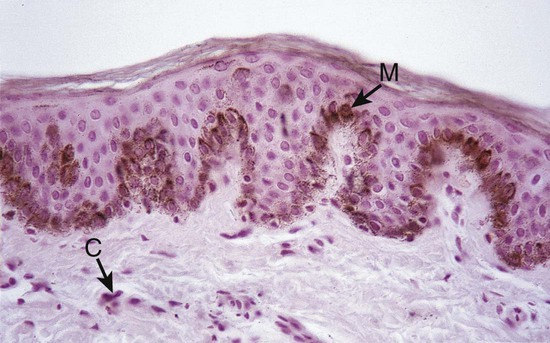
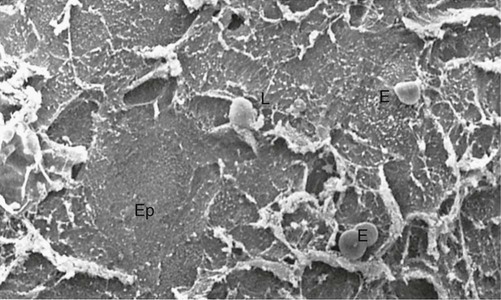
Langerhans cells are dendritic cells located among keratinocytes at all suprabasal levels (Figure 1-12). They belong to the mononuclear phagocyte system (reticuloendothelial system) as modified monocytes derived from the bone marrow. They contain elongated granules, and they are considered macrophages with possible antigenic properties.72 Langerhans cells have an important role in the immune reaction as antigen-presenting cells for lymphocytes. They contain g-specific granules (Birbeck granules), and they have marked adenosine triphosphatase activity. They are found in the oral epithelium of normal gingiva and in smaller amounts in the sulcular epithelium; they are probably absent from the junctional epithelium of normal gingiva.
Merkel cells are located in the deeper layers of the epithelium; they harbor nerve endings, and they are connected to adjacent cells by desmosomes. They have been identified as tactile perceptors.188
The epithelium is joined to the underlying connective tissue by a basal lamina 300 to 400 Å thick and lying approximately 400 Å beneath the epithelial basal layer.147,235,254 The basal lamina consists of lamina lucida and lamina densa. Hemidesmosomes of the basal epithelial cells abut the lamina lucida, which is mainly composed of the glycoprotein laminin. The lamina densa is composed of type IV collagen.
The basal lamina, which is clearly distinguishable at the ultrastructural level, is connected to a reticular condensation of the underlying connective tissue fibrils (mainly collagen type IV) by the anchoring fibrils.183,213,257 Anchoring fibrils have been measured at 750 nm in length from their epithelial end to their connective tissue end, where they appear to form loops around collagen fibers. The complex of basal lamina and fibrils is the periodic acid–Schiff–positive and argyrophilic line observed at the optical level 237,258 (Figure 1-13). The basal lamina is permeable to fluids, but it acts as a barrier to particulate matter.
Structural and Metabolic Characteristics of Different Areas of Gingival Epithelium.
The epithelial component of the gingiva shows regional morphologic variations that reflect tissue adaptation to the tooth and alveolar bone.231 These variations include the oral epithelium, the sulcular epithelium, and the junctional epithelium. Whereas the oral epithelium and the sulcular epithelium are largely protective in function, the junctional epithelium serves many more roles and is of considerable importance in the regulation of tissue health.18 It is now recognized that epithelial cells are not “passive bystanders” in the gingival tissues; rather, they are metabolically active and capable of reacting to external stimuli by synthesizing a number of cytokines, adhesion molecules, growth factors, and enzymes.18
The degree of gingival keratinization diminishes with age and the onset of menopause,199 but it is not necessarily related to the different phases of the menstrual cycle.131 Keratinization of the oral mucosa varies in different areas in the following order: palate (most keratinized), gingiva, ventral aspect of the tongue, and cheek (least keratinized).181
Keratins K1, K2, and K10 through K12, which are specific to epidermal-type differentiation, are immunohistochemically expressed with high intensity in orthokeratinized areas and with less intensity in parakeratinized areas. K6 and K16, which are characteristic of highly proliferative epithelia, and K5 and K14, which are stratification-specific cytokeratins, also are present. Parakeratinized areas express K19, which is usually absent from orthokeratinized normal epithelia.37,205
In keeping with the complete or almost-complete maturation, histoenzyme reactions for acid phosphatase and pentose-shunt enzymes are very strong.47,127
Glycogen can accumulate intracellularly when it is not completely degraded by any of the glycolytic pathways. Thus, its concentration in normal gingiva is inversely related to the degree of keratinization236,285 and inflammation.71,273,276
Oral (Outer) Epithelium.
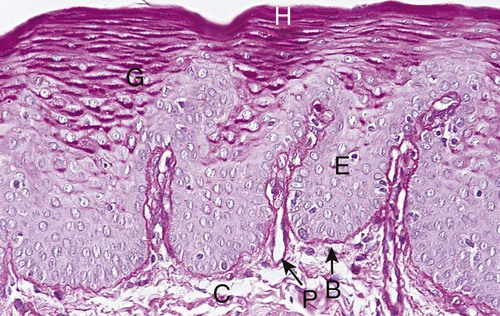
The oral or outer epithelium covers the crest and outer surface of the marginal gingiva and the surface of the attached gingiva. On average, the oral epithelium is 0.2 to 0.3 mm in thickness. It is keratinized or parakeratinized, or it may present various combinations of these conditions (Figure 1-14). The prevalent surface, however, is parakeratinized.32,45,285 The oral epithelium is composed of four layers: stratum basale (basal layer), stratum spinosum (prickle cell layer), stratum granulosum (granular layer), and stratum corneum (cornified layer).
Sulcular Epithelium.
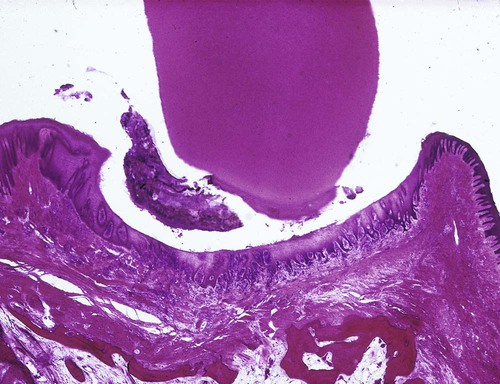
The sulcular epithelium lines the gingival sulcus (Figure 1-15). It is a thin, nonkeratinized stratified squamous epithelium without rete pegs, and it extends from the coronal limit of the junctional epithelium to the crest of the gingival margin (Figure 1-16 ). It usually shows many cells with hydropic degeneration.32
Histochemical studies of enzymes have consistently revealed a lower degree of activity in the sulcular than in the outer epithelium, particularly in the case of enzymes related to keratinization. Glucose-6-phosphate dehydrogenase expresses a faint and homogeneous reaction in all strata, unlike the increasing gradient toward the surface observed in cornified epithelia.127 Acid phosphatase staining is negative,46 although lysosomes have been described in exfoliated cells.148
Despite these morphologic and chemical characteristics, the sulcular epithelium has the potential to keratinize if it is reflected and exposed to the oral cavity44,48 or if the bacterial flora of the sulcus is totally eliminated.50 Conversely, the outer epithelium loses its keratinization when it is placed in contact with the tooth.50 These findings suggest that the local irritation of the sulcus prevents sulcular keratinization.
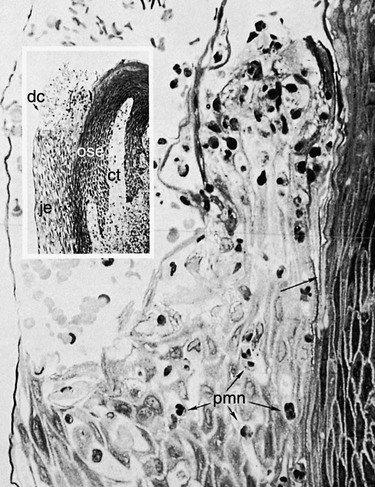
The sulcular epithelium is extremely important; it may act as a semipermeable membrane through which injurious bacterial products pass into the gingiva and through which tissue fluid from the gingiva seeps into the sulcus.267 Unlike the junctional epithelium, however, the sulcular epithelium is not heavily infiltrated by polymorphonuclear neutrophil leukocytes, and it appears to be less permeable.18
Junctional Epithelium.
The junctional epithelium consists of a collarlike band of stratified squamous non-keratinizing epithelium. It is 3 to 4 layers thick in early life, but the number of layers increases with age to 10 or even 20 layers. In addition, the junctional epithelium tapers from its coronal end, which may be 10 to 29 cells wide to 1 or 2 cells wide at its apical termination, which is located at the cementoenamel junction in healthy tissue. These cells can be grouped in two strata: the basal layer that faces the connective tissue and the suprabasal layer that extends to the tooth surface. The length of the junctional epithelium ranges from 0.25 to 1.35 mm (Figure 1-17).
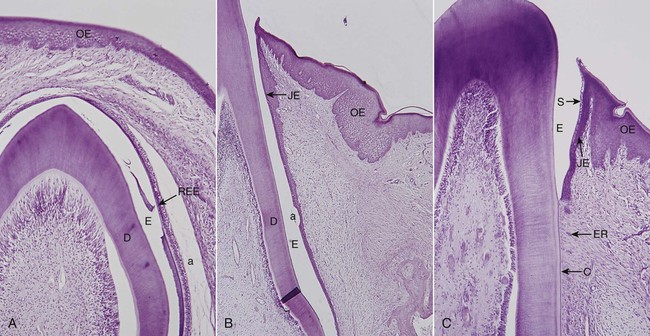
The junctional epithelium is formed by the confluence of the oral epithelium and the reduced enamel epithelium during tooth eruption However, the reduced enamel epithelium is not essential for its formation; in fact, the junctional epithelium is completely restored after pocket instrumentation or surgery, and it forms around an implant.151
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses