Chapter 26
Cancer and Oral Care of the Cancer Patient
Collectively, all cancers combined account for about 23% of deaths in the United States, thereby placing cancer second only to heart disease as a leading cause of death.< ?xml:namespace prefix = "mbp" />
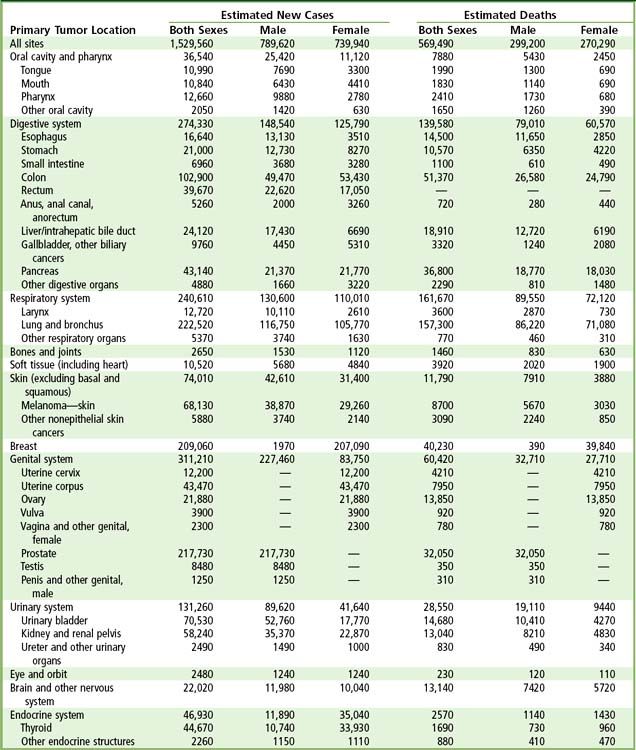
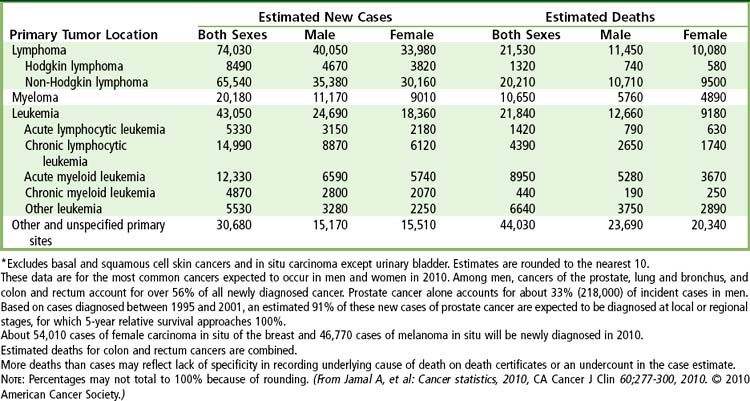
A total of 1.5 million new cancer cases and over 600,000 deaths from cancer are expected in the United States in 2011 (
The death rate from all cancers combined has decreased slightly in the past 10 years.
Because patients diagnosed with cancer are experiencing increased survival as a result of improved diagnostics and advances in antineoplastic therapy, an increased likelihood exists of dentists treating patients in various phases of cancer therapy. For optimum oral health, the dentist should be an integral part of the cancer patient’s health care team. The characteristic clinical course, cancer progression status, treatment modalities, the location of cancer therapy (hospital or outpatient facility), and the likely outcome all will affect the dental treatment plan. Maintenance of proper oral hygiene is critical for limiting local and systemic complications associated with chemotherapy, radiation therapy, and marrow and stem cell transplantation. In addition, dentists have the unique opportunity to reduce the risk of cancer by providing advice regarding cancer screening, a healthy diet, counseling patients as appropriate regarding smoking cessation and risks associated with alcohol consumption, and performing cancer screening procedures.
This chapter focuses on common cancers that may affect patients who require dental care. No attempt is made here to include all cancers; instead, an overview of cancer is presented first, followed by a discussion of common cancers, along with relevant considerations regarding oral care of patients with cancer. A discussion of lymphoma and leukemia can be found in
Definition and Scope of the Problem
Cancer is characterized by uncontrolled growth of aberrant neoplastic cells.
Epidemiology: Incidence and Prevalence
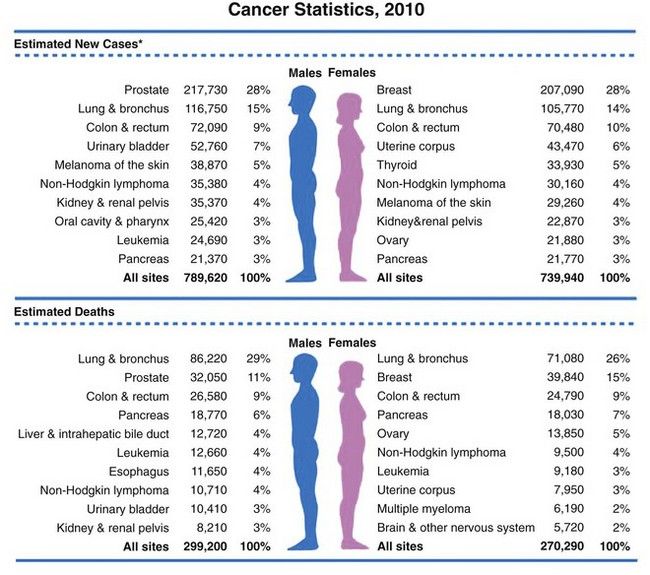
FIGURE 26-1 Ten leading cancer types in estimated new cancer cases and deaths, by gender, United States, 2006. Indicated are the most common cancers that were expected to occur in men and women in 2006. Among men, cancers of the prostate, lung and bronchus, and colon and rectum account for more than 56% of all newly diagnosed cancers. Prostate cancer alone accounts for about 33% (234,460) of incident cases in men. On the basis of cases diagnosed between 1995 and 2001, an estimated 91% of new cases of prostate cancer were expected to be diagnosed at local or regional stages, for which relative 5-year survival approaches 100%.
(From Jamal A, et al: Cancer statistics, 2010, CA Cancer J Clin 60;277-300, 2010.
Etiology and Prevention
Carcinogenesis is a complex multistep process that involves the accumulation of mutations and the loss of regulatory control over cell division, differentiation, apoptosis, and adhesion
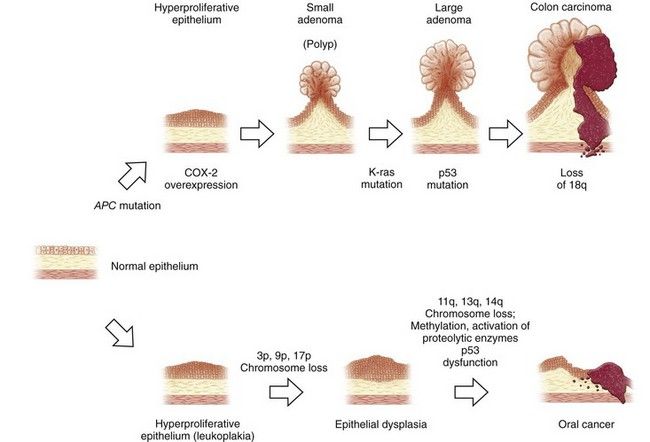
FIGURE 26-2 Carcinogenesis: pathologic sequence in gastrointestinal mucosa. Examples in colon and oral mucosa.
(Adapted from Jänne PA, Mayer RJ: Chemoprevention of colorectal cancer, N Engl J Med 342:1960-1968, 2000.)
TABLE 26-2 Syndromes of Inherited Cancer Predisposition in Clinical Oncology Syndrome
| Syndrome | Mode of Inheritance | Gene(s) |
|---|---|---|
| Hereditary Breast Cancer Syndromes | ||
| Hereditary breast and ovarian cancer syndrome | Dominant | BRCA1 |
| BRCA2 | ||
| Li-Fraumeni syndrome | Dominant | TP53 |
| Cowden’s syndrome | Dominant | PTEN |
| Bannayan-Riley-Ruvalcaba syndrome | Dominant | PTEN |
| Hereditary Gastrointestinal Malignancies | ||
| Hereditary nonpolyposis colon cancer | Dominant | MLH1 |
| MLH2 | ||
| MSH6 | ||
| Familial polyposis | Dominant | APC |
| Hereditary gastric cancer | Dominant | CDH1 |
| Juvenile polyposis | SMAD4/DPC4 | |
| BMPR1A | ||
| Peutz-Jeghers syndrome | Dominant | STK11 |
| Hereditary melanoma–pancreatic cancer syndrome | Dominant | CDKN2A |
| Hereditary pancreatitis | Dominant | PRSS1 |
| Turcot’s syndrome | Dominant | APC |
| MLH1 | ||
| PMS2 | ||
| Familial gastrointestinal stromal tumor | Dominant | KIT |
| Genodermatoses With Cancer Predisposition | ||
| Melanoma syndromes | Dominant | CDKN2A |
| CDK4 | ||
| CMM | ||
| Basal cell cancer, Gorlin’s syndrome | Dominant | PTCH |
| Cowden’s syndrome | Dominant | PTEN |
| Neurofibromatosis 1 | Dominant | NF1 |
| Neurofibromatosis 2 | Dominant | NF2 |
| Tuberous sclerosis | Dominant | TSC1 |
| TSC2 | ||
| Carney’s complex | Dominant | PRKAR1A |
| Muir-Torre syndrome | Dominant | MLH1 |
| MSH2 | ||
| Xeroderma pigmentosum | Recessive | XPA,B,C,D,E,F,G |
| POLH | ||
| Rothmund-Thomson syndrome | Recessive | RECOL4 |
| Leukemia/Lymphoma Predisposition Syndromes | ||
| Bloom’s syndrome | Recessive | BLM |
| Fanconi’s anemia | Recessive | FANCA,B,C |
| FANCA,D2 | ||
| FANCE,F,G | ||
| FANCL | ||
| Ataxia-telangiectasia | Recessive | ATM |
| Shwachman-Diamond syndrome | Recessive | SBDS |
| Nijmegen breakage syndrome | Recessive | NBS1 |
| Canale-Smith syndrome | Dominant | FAS |
| FASL | ||
| Wiskott-Aldrich syndrome | X-linked recessive | WAS |
| Common variable immune deficiency | Recessive | |
| Severe combined immune deficiency | X-linked recessive | IL2RG |
| Recessive | ADA | |
| JAK3 | ||
| RAG1 | ||
| RAG2 | ||
| IL7R | ||
| CD45 | ||
| Artemis | ||
| X-linked lymphoproliferative syndrome | X-linked recessive | SH2D1A |
| Genitourinary Cancer Predisposition Syndromes | ||
| Hereditary prostate cancer | Dominant | HPC1 |
| HPCX | ||
| HPC2/ELAC2 | ||
| PCAP | ||
| PCBC | ||
| PRCA | ||
| Simpson-Golabi-Behmel syndrome | X-linked recessive | GPC3 |
| von Hippel–Lindau syndrome | Dominant | VHL |
| Beckwith-Wiedemann syndrome | Dominant | CDKN1C |
| NSD1 | ||
| Wilms’ tumor syndrome | Dominant | WT1 |
| Wilms’ tumor, aniridia, genitourinary abnormalities, mental retardation (WAGR) syndrome | Dominant | WT1 |
| Birt-Hogg-Dub? syndrome | Dominant | FLCL |
| Papillary renal cancer syndrome | Dominant | MET,PRCC |
| Constitutional t(3;8) translocation | Dominant | TRCB |
| Hereditary bladder cancer | Sporadic | |
| Hereditary testicular cancer | Possibly X-linked | |
| Rhabdoid predisposition syndrome | Dominant | SNF5INI1 |
| Central Nervous System/Vascular Cancer Predisposition Syndromes | ||
| Hereditary paraganglioma | Dominant | SDHD |
| SDHC | ||
| SDHB | ||
| Retinoblastoma | Dominant | RB1 |
| Rhabdoid predisposition syndrome | Dominant | SNF5/INI1 |
| Sarcoma/Bone Cancer Predisposition Syndromes | ||
| Multiple exostoses | Dominant | EXT1 |
| EXT2 | ||
| Leiomyoma/renal cancer syndrome | Dominant | FH |
| Carney’s complex | Dominant | PRKAR1A |
| Werner’s syndrome | Recessive | WRN |
| Endocrine Cancer Predisposition Syndromes | ||
| Multiple endocrine neoplasia 1 | Dominant | MEN1 |
| Multiple endocrine neoplasia 2 | Dominant | RET |
| Familial papillary thyroid cancer | Dominant | Multiple loci |
Adapted from Garber JE, Offit K: Hereditary cancer predisposition syndromes, J Clin Oncol 23:276-292, 2005.
The aggregation of cancer in a family can be due to genetic or nongenetic causes, the former through mendelian (single-gene mutation) or nonmendelian (polygenic or multifactorial) inheritance of genes that predispose to cancer and the latter related to common exposure to carcinogenic agents or lifestyle, or simple coincidence. The modern understanding of familial aggregation of cancer has required increasingly sophisticated epidemiologic and statistical methods in combination with genetic concepts and technologies.
Although mendelian inheritance accounts for a small minority of all cancers, mutations that predispose to cancer have provided some of the most penetrating insights into the understanding of the genetic basis of normal as well as abnormal development; these mutations manifest the classical recessive or dominant modes of inheritance. Nonmendelian inheritance, which also plays a major role in the overall incidence of cancer, has been more difficult to characterize. In addition, the interaction of mutated genes with the environment adds another level of complexity in deciphering the role of genetics of cancer in individual patients as well as in families.
At least three to six somatic mutations are needed to transform a normal cell into a malignant cell. Acquired mutations can arise from exposure to hazardous chemicals and pathogens that lead to activation of oncogenes, inactivation of tumor suppressor genes (pRb and TP53), and chromosomal abnormalities (translocations, deletions, insertions). The accumulation of these abnormalities leads to a cell that becomes functionally independent and aggressive. Natural killer cells provide surveillance for cancerous cells. Reduction in numbers or function of natural killer cells, which occurs during immunosuppression, increases the risk for cancer.
National efforts currently focus on the reduction or elimination of factors known to be associated with cancer. Recommendations from the American Cancer Society (ACS) are to minimize exposure to tobacco smoke and to environmental and occupational carcinogens (e.g., asbestos fibers, arsenic compounds, chromium compounds, pesticides); decrease intake of fat and exposure to ultraviolet light; moderate the intake of alcohol; obtain an adequate intake of dietary fiber and antioxidants (vitamins C and E, selenium); and perform moderate levels of physical activity.
Pathophysiology and Complications
The loss of regulatory control in a cell destined to become a cancer cell results in a series of pathologic changes that eventuate in hyperproliferative epithelium, dysplasia, and finally carcinoma. Dysplastic tissue is characterized by atypical cell proliferation, nuclear enlargement, failure of maturation, and differentiation short of malignancy (see
Cytogenetic studies of various leukemias established four cardinal attributes of genetic change in cancer: (1) Specific or nonrandom chromosomal changes may characterize individual cancer types; (2) tumor genomes are genetically unstable and subject to continuing change, a feature now recognized as genomic instability; (3) all cells in a given tumor trace back to a single progenitor cell and therefore are clonal; and (4) tumor progression often is associated with additional specific or nonrandom chromosomal changes, presumably “selected” from the genomic instability, in subpopulations of tumor cells that lead clonal diversity and evolution. Chromosomal changes are of many types, the most common being gain of an entire chromosome (aneuploidy) or a region of it (duplication), loss of an entire chromosome (monosomy) or a region of it (deletion), translocation or inversion (rearrangement), and amplification (
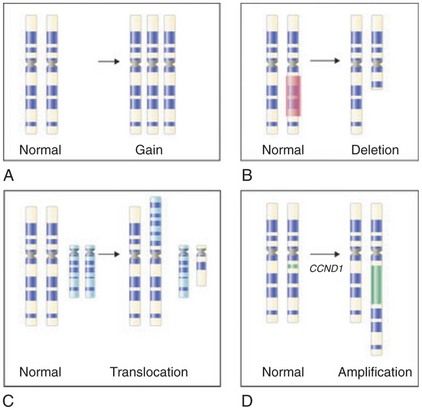
FIGURE 26-3 Common cytogenetic changes in cancer. The chromosome (at metaphase) is traditionally distinguished by its short and long arms separated by a centromere. Stylized bands (dark and light stripes along the length of the chromosome) produced by special treatments are also shown. The abnormality (right) and the corresponding normal image of the chromosome are illustrated in each panel. A, Gain of a chromosome leading to aneuploidy. B, Deletion of a chromosomal segment from one of the two homologues. C, Translocation showing exchange of segments between nonhomologous chromosomes. D, Amplification, with an increase in a region of a chromosome by replicating many times in place.
(From Goldman L, Ausiello D, editors: Cecil textbook of medicine, ed 23, Philadelphia, 2008, Saunders.)
Malignant cells exhibit antigenic, karyotypic, biochemical, and membrane changes that cause loss of contact inhibition, changes in chromosomal morphology, and increased permeability. Malignant tumors lack cell cycle control and replicate rapidly, becoming clinically detectable after about 30 cell doublings, when the mass contains about 109 cells (1 g). A three-log increase to 1012 cells produces a tumor that weighs 1 kg and often is lethal. After reaching clinically detectable size, tumors slow in growth as they reach anatomic boundaries and begin to outgrow their blood supply. Malignant tumors overcome the limitation of anatomic boundaries by losing cell adherence and by metastasizing. Metastasis is a distinct form of cancerous spread that occurs when malignant cells enter blood or lymphatic vessels and travel to distant sites. Metastasis is related to factors produced by tumors cells that allow individual cells to invade tissues and endothelium. It often results in end-organ failure and death.
Clinical Presentation
Screening
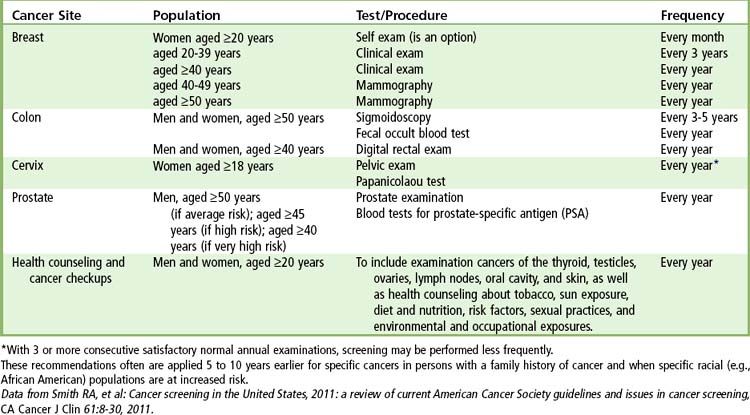
Each year the ACS publishes a summary of its recommendations for early cancer detection. Obviously, the earlier any form of cancer is diagnosed, the more expeditiously and effectively it can be treated in order to minimize adverse outcomes: morbidity and mortality.
Signs and Symptoms
Cancers often manifest as a palpable mass that increase in size over time. Preceding the development of the tumor are subtle changes that are dependent on the anatomic site involved and the cell type of origin. Initial features can include a change in surface color, a lump, enlarged lymph node, or altered organ function. Symptoms include pain and paresthesia. Tumors permitted to increase in size often result in a reddened epithelial surface (due to increased blood vessels) that ulcerates.
Staging
Most cancers are assigned a stage (I, II, III, or IV) by the medical team on the basis of the size of the tumor and how far it has spread (

Box 26-1
International Tumor-Node-Metastasis (TNM) System of Classification and Staging of Oral Carcinomas
T: Tumor Size
N: Regional Lymph Node Involvement
Stage Classification
| 0 (carcinoma in situ) | TIS, N0, M0 |
| I | T1, N0, M0 |
| II | T2, N0, M0 |
| III | T3, N0, M0 or T1, T2 or T3, N1, M0 |
| IVA | T4, N0, M0 or T4, N1, M0 or any T, N2, M0 |
| IVB | Any T, N3, M0 |
| IVC | Any T, any N, M1 |

Laboratory Findings
The diagnosis of cancer is dependent on microscopic examination of an adequate sample of tissue taken from the lesion (

Box 26-2 Microscopic Criteria for Malignancy
Cytoplasm Scant cytoplasm, increased nucleus to cytoplasm ratio, tight molding of cytoplasmic membrane around nucleus
Nucleus Enlargement with variation in size, irregular membrane with sharp angles, hyperchromasia, irregular chromatin distribution with clumping, prominent nucleoli, abundant or abnormal mitotic figures
Relationships Variation in cell size and shape, abnormal stratification, decreased cohesiveness

Medical Management
Treatment strategies for cancer are based on eliminating fast multiplying cancer cells without killing the host. Therapeutic modalities include surgery; irradiation (by external beam or implants); regimens based on cytotoxic, chemotherapeutic, and endocrine drugs; and possibly stem cell or bone marrow transplantation. Surgery often is used when anatomy permits to debulk a tumor or if the cancer is limited in size. Radiation therapy (often at doses greater than 50 grays [Gy]
TABLE 26-4 Chemotherapy Drugs of Choice for Common Cancers
| Cancer | Drugs of Choice |
|---|---|
| Breast | Risk reduction: Tamoxifen |
| Adjuvant: Doxorubicin + cyclophosphamide ± fluorouracil followed by paclitaxel; cyclophosphamide + methotrexate + fluorouracil; tamoxifen for receptor-positive and hormone-responsive tumors | |
| Metastatic: Doxorubicin + cyclophosphamide ± fluorouracil; cyclophosphamide + methotrexate + fluorouracil | |
| Tamoxifen or toremifene for receptor-positive and/or hormone-responsive tumors | |
| Paclitaxel + trastuzumab for tumors that overexpress HER2 protein | |
| Cervix | Locally advanced: Cisplatin ± fluorouracil |
| Metastatic: Cisplatin; ifosfamide with mesna; bleomycin + ifosfamide with mesna + cisplatin | |
| Colorectal | Adjuvant: Fluorouracil + leucovorin |
| Metastatic: Fluorouracil + leucovorin + irinotecan | |
| Head and neck | Cisplatin + fluorouracil or paclitaxel |
| Kaposi sarcoma | Liposomal doxorubicin or daunorubicin; doxorubicin + bleomycin + vincristine |
| Leukemia and lymphoma | See |
| Liver | Hepatic intraarterial floxuridine, cisplatin, doxorubicin or mitomycin |
| Lung | |
| Non–small cell | Paclitaxel + cisplatin or carboplatin; cisplatin + vinorelbine; gemcitabine + cisplatin; cisplatin or carboplatin + etoposide (PE) |
| Small cell | |
| Melanoma | Adjuvant: Interferon alfa |
| Metastatic: Dacarbazine | |
| Multiple myeloma | Melphalan or cyclophosphamide + prednisone; vincristine + doxorubicin (Adriamycin) + dexamethasone (VAD) |
| Prostate | Gonadotropin-releasing hormone (GnRH) agonists (leuprolide or goserelin) ± antiandrogen (flutamide, bicalutamide, or nilutamide) |
| Renal | Interleukin-2 |
Adapted from Drugs for cancer, Med Lett Drugs Ther 42:83-92, 2000.
Breast Cancer
Breast cancer is the most common type of cancer in the United States, with 98% of cases occurring in women. In 2010, approximately 207,000 cases of breast cancer were reported in the United States, with about 40,000 persons dying of the disease in that year.
Breast cancer often is detected as a lump in the breast with or without nipple discharge, breast skin changes and breast pain. Mammography detects the mass in only 75% to 85% of patients (
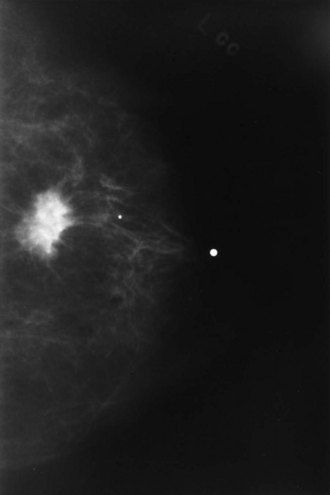
FIGURE 26-5 Mammogram showing a radiodense area in the breast suggestive of a malignancy that should be recommended for biopsy.
(Courtesy A.R. Moore, Lexington, Kentucky.)
Treatment of breast cancer depends on the histologic type of cancer and stage. Cellular markers such as the Her2/neu molecule (target of drug herceptin) and the sodium-iodide symporter (NIS) aid in the diagnosis and treatment planning. Lumpectomy (when the tumor is less than 5 cm) or lumpectomy plus radiotherapy is preferred over radical mastectomy. Axillary node dissection is performed if the regional sentinel node is positive for malignancy. Hormone therapy (tamoxifen) and chemotherapy combined with local therapy is recommended when invasive carcinoma exceeding 1 cm in diameter or axillary lymph nodes are positive. The combination of fluorouracil, doxorubicin, and cyclophophamide usually is administered for 4 to 6 months, given at 3- to 4-week intervals. At present, metastatic breast cancer is incurable. Accordingly, the ACS recommends a mammogram and professional clinical examination every year for women 40 years of age and older (
Cervical Cancer
Cancer of the uterine cervix occurred in nearly 12,000 women in the United States in 2010, and more than 4000 women died of the disease.
Human papillomaviruses (HPVs), which are epitheliotropic sexually transmitted DNA viruses, are the major etiologic agent of cervical carcinogenesis. These viruses dysregulate the cell cycle and tumor suppressor genes (TP53 and pRb) through overexpression of viral early genes E6 and E7. Certain HPV strains (HPV serotypes 16, 18, 45, and 56) are classified as high-risk types, because they are associated with a majority of cases. HPV types 30, 31, 33, 35, 39, 51, 52, 58, and 66 are classified as intermediate oncogenic risk. In addition to viral infection, chronic cigarette smoking, multiple sexual partners, and immunosuppression increase the risk of cervical cancer
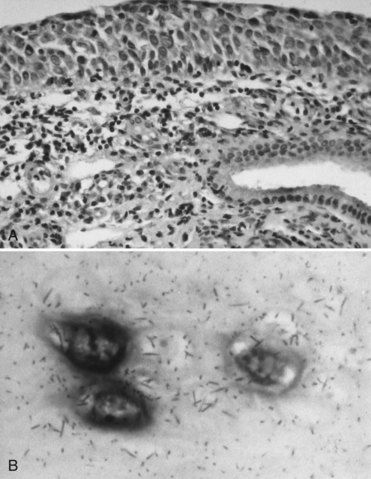
FIGURE 26-6 A, Biopsy specimen revealing cancerous epithelium of the uterine cervix (hematoxylin and eosin stain). B, Human papillomavirus DNA detected in cervical epithelium by in situ hybridization.
(Courtesy Dr. Michael Cibull, Lexington, Kentucky.)
Cervical cancer typically has a long asymptomatic period before the disease becomes clinically evident. The cancer classically manifests in women who are between 40 and 60 years of age. The earliest preinvasive changes are diagnosed by Pap smear. Further evaluation is made by colposcopy and colposcopy-directed biopsy. If neoplastic cells penetrate the underlying basement membrane of the uterine cervix, widespread dissemination can occur. Metastases often affect renal tissues, resulting in ureteral obstruction and azotemia. Treatment is based on the stage of the disease and involves hysterectomy in the early stages and radiation therapy for disease that extends to or invades local organs. The 5-year survival rate is relatively high (see
The ACS recommends that a Pap smear and professional pelvic examination be performed in women at the onset of sexually activity or at 18 years of age. Because cervical cancer is associated with immunosuppression, the Centers for Disease Control and Prevention (CDC) advises all women who are seropositive for human immunodeficiency virus (HIV) to receive semiannual screening beginning the first year after diagnosis. Health care providers may elect to screen less often when three annual examinations in a row are negative.
Colorectal Cancer
Cancer of the large bowel (colon and rectum) is the most common malignancy of the gastrointestinal tract and overall the fourth most common cancer of persons living in the United States. This cancer was diagnosed in approximately 160,000 persons in the United States in 2010, and nearly 60,000 people died of the disease in that year.
The vast majority of colorectal cancers are adenocarcinomas (
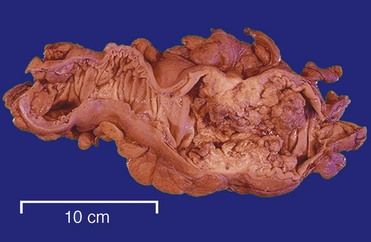
FIGURE 26-7 Destructive effects of colon cancer.
(From Klatt ED: Robbins and Cotran atlas of pathology, ed 2, Philadelphia, 2010, Saunders.)
Colorectal cancer often is not diagnosed until age 40 and increases in incidence after age 50. Risk rises sharply by age 60 and doubles every decade until it peaks at age 75 years. Spread is by direct extension through the bowel wall and invasion of adjacent organs by lymphatics and the portal vein to the liver. The major signs and symptoms of colorectal cancer are rectal bleeding, abdominal pain, and change in bowel habits (constipation). Presenting symptoms may include those referable to invasion of adjacent organs (kidney, liver, vagina).
Colonoscopy is the preferred approach for evaluating a patient for colorectal cancer. This approach permits tissue and brush biopsy to be performed. Staging of the patient is aided by endoscopic ultrasonography and computed tomography (CT) scanning. Surgical excision is the treatment of choice with lesions encroaching the distal 5 cm of the colon, resulting in colostomy. Radiation therapy is used for treatment of rectal and anal cancer. Chemotherapy (fluorouracil and leucovorin for up to 6 months or, more recently, topoisomerase I inhibitors [camptothecins] and oxaliplatin) are used when metastatic spread occurs. Liver metastases have been treated with hepatic arterial therapy using implantable pumps and injection ports to deliver chemotherapeutic agents.
The poor prognosis with advanced colorectal cancer (stage III or IV) emphasizes the need for annual screening of at-risk adults. Digital rectal examination, fecal occult blood test, stool DNA testing, sigmoidoscopy, colonoscopy, and barium enema with air contrast are the screening procedures for colorectal cancer. The ACS recommends that screening start at age 50 for both men and women and even earlier if a family history exists, especially among first-degree relatives of colorectal cancer, preexisting inflammatory bowel disease, a personal history of colorectal cancer or adenomatous polyp, or a family history of hereditary colorectal cancer syndromes (e.g., familial adenomatous polyposis, Peutz-Jeghers syndrome, Gardner’s syndrome). Digital rectal examination and a test for occult blood should be performed once a year. Sigmoidoscopy is recommended every 5 years and colonoscopy every 10 years. A barium enema can be performed in place of the sigmoidoscopy and colonoscopy.
Lung Cancer
Lung cancer is the cause of 14% of cancer cases and is the leading cause of cancer deaths (almost 157,000 deaths annually) in the United States (see
Histologically, lung cancers are divided into two groups. About 80% are non–small cell lung cancers (large cell undifferentiated 10%; squamous cell carcinoma [SCC] 30%; and adenocarcinoma 40%) (
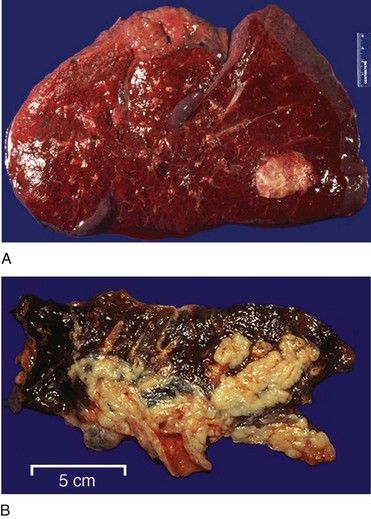
FIGURE 26-8 Large cell undifferentiated carcinoma infiltrating the entire lung shown in cross section.
(From Klatt ED: Robbins and Cotran atlas of pathology, ed 2, Philadelphia, 2010, Saunders.)
Lung cancer is a clinically silent disease until late in its course. Tumors that grow locally can produce a cough or change the nature of a chronic cough or manifest as dyspnea on exertion. Cancers that invade adjacent structures can produce chest pain and dyspnea, hemoptysis or produce syndromes (e.g., Horner’s syndrome) from disruption of nerves in the chest and neck or endocrine, cutaneous, or neurologic manifestations. Metastases to the brain, bone, adrenal gland, and liver produce features associated with malfunction of these organs and lymphadenopathy. With advanced disease, patients present with anorexia, weight loss, weakness, and profound fatigue.
Unfortunately, so far there is not any lung cancer screening test that has been shown to prevent people from dying of this disease. The use of chest x-ray imaging and sputum cytology (evaluating phlegm microscopically for abnormal cells) has been studied for several years. The recently updated studies have not yet yielded any value in screening programs for the early detection of lung cancer. Lung cancer screening is not recommended even for persons at high risk such as smokers.
The diagnosis of lung cancer is made by imaging studies, bronchoscopy, bronchial washings, brush and tissue biopsies, and histologic examination of the cells and tissue. Stage I and stage II non–small cell lung cancers are treated by surgical resection. Radiotherapy is used for more advanced non–small cell lung cancers and when patients with stage I or II disease refuse or are medically unfit for surgery. Chemotherapy using two or three agents (e.g., cisplatin, carboplatin, etoposide, vinblastine, vindesine) is employed in combination with radiotherapy for stage III and stage IV non–small cell lung cancers. Chemotherapy is the mainstay of treatment for small cell lung cancer. Adjuvant radiotherapy is used in patients with limited disease. Stage I lung cancer and stage II squamous cell lung cancers are associated with 5-year survival rates of more than 50%. The current 5-year survival rate for all stages of lung cancer is just 15.8%.
Prostate Cancer
Prostate cancer is the second most common cancer (approximately 234,000 cases per year) and the most common cancer of men in the United States (see
At present, the etiologic factors for prostate cancer remain unknown. High dietary fat intake and mutations in chromosome 1 (1q24-25) and X (Xq27-28) appear to increase the risk for prostate cancer. Overexpression of the c-myc oncogene also is commonly detected in solid tumors such as prostate cancer.
More than 90% of all prostate carcinomas are adenocarcinomas. They typically arise at multiple locations within the gland. Cancer of the prostate produces few signs and symptoms other than problems in urination (hesitancy, decreased force of urination) that, if present, occur late in the course of the disease. Thus screening procedures are paramount to the successful management of this disease. Methods used to screen for prostate cancer include the digital rectal examination (DRE) in combination with blood tests for prostate-specific antigen (PSA), and endorectal ultrasound imaging (
• PSA value greater than 10 ng/mL
• A positive DRE (palpable nodule or abnormality); even if the PSA value is less than 4 ng/mL, a positive DRE represents about 25% of all prostate cancer
• PSA value between 4 and 10 ng/mL, a negative DRE
• PSA value less than 4 ng/mL, a negative DRE, and a PSA value that has increased from 1 year to the next by 0.75 ng/mL (PSA velocity) or more
Radionuclide scanning or pelvic magnetic resonance imaging (MRI) is recommended for men diagnosed with prostate cancer with a PSA greater than 10 ng/mL to determine the extent of the disease. Metastasis occurs by lymphatic or hematogenous d/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses