The rapidly evolving field of vascular anomalies combines the expertise of several surgical, radiologic, and medical specialists. In the past, the field was obscured by a cloud of confusing descriptive and histologic words. Improper terminology and classifications led to incorrect diagnosis, inappropriate treatment, and ill-conceived research endeavors. Vascular anomalies were often called “ hemangioma ” and specified by adjectives such as “capillary,” “strawberry,” “cavernous,” “central,” “venous,” etc. Unfortunately, the generic label “ hemangioma ” was applied to many types of vascular lesions, both congenital and acquired, with different etiologies and clinical presentations.
In 1982 Mulliken and Glowacki analyzed the cellular features of the various vascular lesions seen in infancy and childhood and correlated their findings with physical examination and natural history. They concluded that, on the basis of cellular kinetics, there are two major types of vascular anomalies: infantile hemangioma (IH) and vascular malformations. In 1996 The International Society for the Study of Vascular Anomalies adopted an expanded schema by categorizing vascular anomalies into tumors and malformations . In modern medical usage, the Greek nominative suffix “-oma” refers to a lesion that arises by up-regulated cellular growth. Hence, the word hemangioma denotes a benign vascular tumor, almost exclusively occurring in infancy. There are other vascular tumors with borderline or intermediate malignant features specified as hemangioendotheliomas. The least common are malignant vascular tumors, angiosarcomas, and lymphangiosarcomas.
Vascular malformations arise as the result of abnormal embryonic development. They are subdivided anatomically and rheologically into slow-flow lesions (i.e., capillary malformation [CM], lymphatic malformation [LM], and venous malformation [VM]) and fast-flow lesions (i.e., arterial malformations, such as aneurysm, ectasia, stenosis, fistula, and arteriovenous malformation [AVM]) ( Box 29-1 ).
More than 90% of vascular anomalies can be differentiated by history and physical examination. About one third of IHs are nascent at birth; two thirds appear postnatally. Rapid growth in early infancy is the defining feature. In contrast, many vascular malformations are noted at birth, and they expand commensurately with the child. Radiologic examination, ultrasonography (US), and magnetic resonance imaging (MRI) are commonly used to confirm the clinical diagnosis, document the extent and flow characteristics, and search for other vascular lesions. A biopsy is mandatory if there is equivocation about the clinical or radiologic diagnosis.
Histologically, hemangiomas demonstrate endothelial hyperplasia and glucose transporter-1 protein (GLUT1) immunopositivity, whereas vascular malformations have slow endothelial turnover and are GLUT1 negative.
There are no specific hematologic abnormalities associated with IHs. Slow-flow vascular malformations, specifically venous and lymphaticovenous types, exhibit stasis, causing localized and disseminated intravascular coagulopathy.
IHs rarely cause skeletal hypertrophy or deformation of nearby bone by a mass effect, whereas slow-flow vascular malformations can cause bony deformation, and fast-flow lesions typically cause osteolysis.
Since the single case report in 1940, the double eponym Kasabach-Merritt has been used to include profound thrombocytopenia, petechiae, and bleeding in association with a “giant hemangioma. ” Unfortunately, the eponym continues to be commonly applied to the disseminated intravascular coagulopathy seen with slow-flow anomalies. Kasabach-Merritt coagulopathy is not a syndrome, rather it is a phenomenon that occurs only with more invasive vascular tumors, kaposiform hemangioendothelioma (KHE), and tufted angioma (TA).
INFANTILE HEMANGIOMA
The term hemangioma should be restricted to the endothelial tumor of infancy that displays a unique biologic behavior of rapid growth and slow regression.
This most common tumor of infancy occurs in 4% to 10% of Caucasian infants, it is less frequent in dark-skinned infants. By age 1 year, approximately 10% to 12% of Caucasian children have a hemangioma. Females are more affected with a gender ratio of 2.4 : 1. Hemangiomas occur most frequently in the head and neck region (60%). There is an increased incidence of 23% in premature infants weighing less than 1200 g. Eighty percent of cutaneous hemangiomas are single, and 20% are multiple. Multiple cutaneous lesions often are associated with hemangiomas in other organ systems, especially liver, brain, gastrointestinal tract, and rarely in the lung.
The typical infantile hemangioma (IH) is usually not evident at birth, although in 30% of patients, there may be a precursor lesion, such as a small macular red spot, pale area, telangiectasia, or ecchymotic lesion. IHs appear during the first to fourth weeks of life and are followed by rapid postnatal growth, peaking by 5 to 6 months (proliferating phase). After the first year, the IH stabilizes and for a time grows at the same rate as the child and then slowly regresses (involuting phase). Proliferation and involution are not distinct stages in the life cycle, rather proliferation continues as involution slowly begins to predominate. As the hemangioma regresses, it becomes softer and its erythematous color changes to a gray hue. Complete resolution (involuted phase) occurs in more than 50% of children by age 5 and in more than 70% by the age of 7, with continued improvement until ages 10 to 12 ( Figure 29-1 ). After involution is complete, there may be redundant atrophic skin, yellow discoloration or scarred patches if there was previous ulceration, residual fibrofatty tissue, and telangiectasia ( Figure 29-2 ).
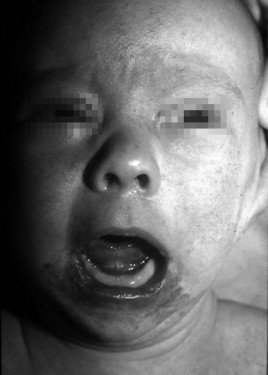
The three phases of the IH’s life cycle can be distinguished clinically, microscopically, and immunohistochemically. In the proliferating phase, the tumor is composed of plump, rapidly dividing endothelial cells that form tightly packed sinusoidal channels. The endothelial cells express phenotypic markers of activated and mature endothelium. Angiogenic proteins (e.g., basic fibroblast growth factor [bFGF] and vascular endothelial growth factor [VEGF]) and enzymes involved in remodeling of extracellular matrix (e.g., type IV collagenase and high-molecular-weight matrix metalloproteinases) are prominent in proliferating hemangiomas and diminish to normal levels during regression. In the involuting phase, there is decreasing endothelial proliferation, increasing apoptosis, preponderance of stromal cells (e.g., mast cells, fibroblasts, and macrophages), and the first evidence of fibrofatty replacement of the tumor. After regression is complete, all that remains are a few tiny capillary-like feeding vessels (encased in multilaminated basement membrane) and draining veins surrounded by islands of fibrofatty tissue admixed with dense collagen and reticular fibers.
If the tumor grows in the superficial dermis, the skin becomes raised, crimson in color, and firm and rubbery to palpation. However, if the hemangioma develops in the lower dermis and subcutis, the lesion may appear slightly raised with a bluish hue or the overlying skin can be smooth and of normal color. These deeper lesions, once labeled “cavernous hemangiomas,” often are not detected until 3 to 4 months of age. Both superficial and deep lesions are firm to palpation and exhibit the characteristic to-and-fro murmur on continuous wave Doppler examination. During the proliferative phase, they can become ulcerated and infected. Multifocal hemangiomas can involve visceral organs (e.g., liver, gastrointestinal tract, brain, and lung) and if large can divert significant intravascular volume, resulting in high-output cardiac failure.
ASSOCIATED MALFORMATIVE ANOMALIES
IHs in the head and neck can arise in association with structural anomalies, which are not necessarily vascular in nature. This was not well appreciated until these various malformations were compiled as the acronym PHACE: P osterior fossa anomalies, H emangioma of the face, A rterial abnormalities, C ardiac defects and coarctation, and E ye anomalies. PHACE association occurs in a spectrum; the female preponderance is 9:1, higher than in isolated IH. The facial hemangioma typically manifests at birth as a diffuse (regional) reticular stain in the frontal and maxillary areas that tends to become plaquelike and pebbly. If the cheek and mandibular areas are involved (“beard distribution”), there also may be an obstructing laryngeal (subglottic) hemangioma. PHACE-associated anomalies also can occur with focal and multifocal hemangiomas. PHACE association should be promptly diagnosed by MRI or magnetic resonance angiography (MRA). Vascular anomalies include: persistent embryonic intracranial and extracranial arteries, hypoplasia or absence of the ipsilateral carotid or vertebral vessels, aneurysmal dilatation of the carotid artery, and coarctation and right-sided aortic arch. Cerebrovascular occlusive changes, resembling moyamoya, can develop in these infants. Seizure and stroke, secondary to cerebral infarction, can occur in infancy, and progressive stenosis and tortuosity can cause neurologic sequelae in childhood and adolescence. The original acronymic designation PHACE did not include the possibility of sternal nonunion or supraumbilical raphe; these were included later by adding S to make PHACES.
CONGENITAL HEMANGIOMA
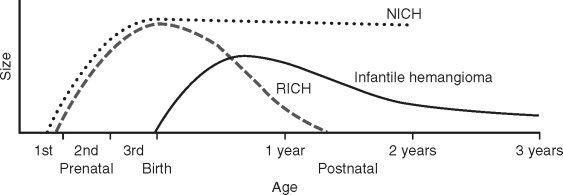
There are rare vascular tumors that occur fully grown at birth and do not exhibit the expected postnatal course and life cycle of the common IH. These are defined as congenital hemangiomas, and there are two subtypes: rapidly involuting congenital hemangioma (RICH) and the noninvoluting congenital hemangioma (NICH) (see Figure 29-1 ). Both RICHs and NICHs have an equal sex ratio. RICH is raised with a characteristic red-violaceous color, central telangiectasias, and frequently with a peripheral pale halo ( Figure 29-3 ). There may be superficial ulceration, central nodularity, or scar. Rapid arteriovenous shunting can simulate an AVM. Transient thrombocytopenia can also occur; however, the hematologic profile is more that of consumptive coagulopathy (i.e., it is not a Kasabach-Merritt phenomenon). Platelet levels rise rapidly as the tumor regresses. RICH commonly occurs on the trunk and extremities, but also in the head and neck and the liver. RICH involutes rapidly during the first few weeks or months of life.
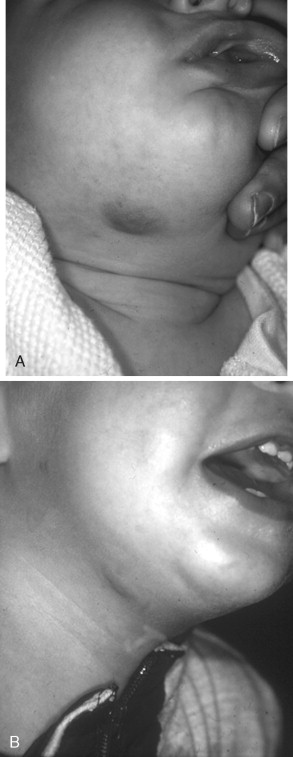
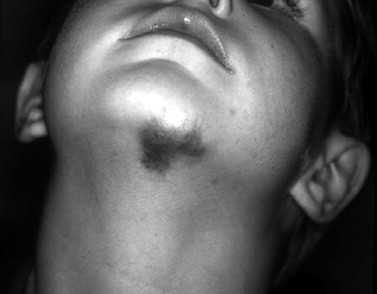
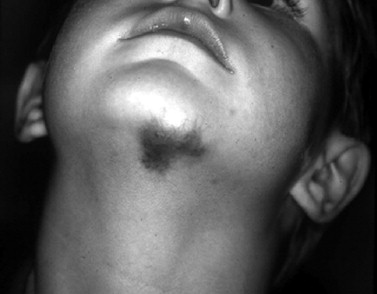
NICHs are typically ovoid, macular, or slightly raised; light gray in color with prominent coarse telangiectasias; and warm on palpation ( Figure 29-4 ). There is a predisposition for the mandibular border. An NICH is typically a wellcircumscribed lesion, averaging 5 to 6 cm in diameter. An NICH is usually not diagnosed until later in childhood because it was mistaken for common IH or presumed to be a small AVM. NICH can occur as a large lesion in the cervical and occipital areas. Sometimes lesions in this location expand during adolescence and exhibit polypoid excrescences.
RICHs and NICHs have overlapping radiologic and histologic features with IHs; however, curiously, neither type of congenital tumor immunostains for glucose transporter-1 protein (GLUT1). This raises the question of whether these congenital hemangiomas are variations in the hemangiomatous spectrum or entirely different tumors that arise in fetal life. There are more similarities than dissimilarities between IHs, RICHs, and NICHs. Furthermore, RICHs and NICHs can coexist with IHs.
MANAGEMENT
Observation and waiting for involution is appropriate for small hemangiomas that cause no harm or disfigurement. Spontaneous ulceration has been reported to occur in up to 20% of cutaneous hemangiomas ; this most frequently involves lesions on the lips, perineum, anogenital area, and extremities ( Figure 29-2 ). An ulcerated hemangioma can become infected or destroy a lip, nose, or eyelid. For small superficial ulcerations, treatment includes cleansing with application of hydrated petrolatum dressing or a topical antibiotic. Scarring is worse in ulcerated areas compared with nonulcerated areas following regression. There is some evidence to suggest that pulsed dye laser therapy can be effective for ulcerated hemangiomas. Nevertheless, pulsed dye laser therapy for proliferating and early involuting hemangiomas is not indicated because the laser cannot penetrate deep enough into the tumor to be effective. Laser application during proliferation of the hemangioma can cause ulceration, hypopigmentation, and scarring. Laser therapy does not alter the life cycle of the hemangioma. A pulsed dye laser is effective for photocoagulation of the residual telangiectasias that persist after involution.
Pharmacologic therapy is indicated if a hemangioma causes destruction, distortion, or obstruction—for example, a proliferating hemangioma producing subglottic narrowing and distortion of the growing cornea, resulting in astigmatic amblyopia or blockage of the visual axis, causing deprivation amblyopia and failure to develop binocular vision. Subglottic hemangioma can be life threatening. The symptoms are hoarseness and biphasic stridor, generally manifesting between 4 and 12 weeks of age. Approximately 50% of these infants have a cutaneous hemangioma in a beardlike distribution. A tracheostomy may be necessary if the lesions are large or unresponsive to pharmacologic therapy. Other alternatives include a carbon-dioxide laser (for unilateral lesions) and open resection.
Intralesional steroids are useful for small lesions on the face (less than 2 cm in diameter). Systemic steroids, vincristine, and interferon-α should be used only in infants with a hemangioma that (1) is rapidly growing and seriously distorting facial features; (2) causing recurrent bleeding, ulceration, or infection; and (3) impinging on critical anatomic structures. Endangering and life-threatening complications, such as high-output cardiac failure with a large tumor or multiple intrahepatic tumors, occur in 10% of hemangiomas. The responsiveness to corticosteroids, the first-line treatment, is as high as 85%, with stabilization of growth or accelerated regression. Indications for second-line therapy (vincristine, interferon-α) are failure of response to corticosteroids, contraindications to prolonged systemic corticosteroids, and complications of corticosteroids.
Resection is a consideration in all three phases of the hemangioma life cycle. Indications for excision in infancy include: obstruction of a vital structure, recurrent bleeding, and ulceration. Resection during early involution can be carried out if excision is inevitable because of postulceration scarring, expanded skin, or high possibility of fibrofatty residuum and if the scar is easily concealed or if staged excision will be necessary. Indications for resection during late childhood include damaged skin, excess skin, abnormal contour (fibrofatty residuum), distortion, and loss of tissue. Resection of a facial hemangioma, in any of the phases of its life cycle, requires careful planning. The conventional method is excision in a lenticular format with linear closure in the axis of the relaxed cutaneous tension lines, either as a single or staged procedure. This technique is applicable in some locations, such as the lips, eyelids, neck, and sometimes the scalp. Most hemangiomas are spherical, and lenticular excision (to minimize “dog ears”) causes central tension that flattens a convex area, such as the cheek or forehead, or may cause distortion of nearby structures. Hemangiomas act as a tissue expander; therefore circular excision and purse-string closure should always be the first consideration. This technique converts a circular lesion into a small circular or ellipsoid scar. Thereafter, a second excision can be either circular or lenticular. The result is the smallest possible scar with minimal distortion of surrounding structures.
RICH often leaves behind loose skin and prominent draining veins. Resection may be indicated in an end-stage RICH. NICH can be resected, and diagnosis is established; usually the indication is cosmetic.
CONGENITAL HEMANGIOMA
There are rare vascular tumors that occur fully grown at birth and do not exhibit the expected postnatal course and life cycle of the common IH. These are defined as congenital hemangiomas, and there are two subtypes: rapidly involuting congenital hemangioma (RICH) and the noninvoluting congenital hemangioma (NICH) (see Figure 29-1 ). Both RICHs and NICHs have an equal sex ratio. RICH is raised with a characteristic red-violaceous color, central telangiectasias, and frequently with a peripheral pale halo ( Figure 29-3 ). There may be superficial ulceration, central nodularity, or scar. Rapid arteriovenous shunting can simulate an AVM. Transient thrombocytopenia can also occur; however, the hematologic profile is more that of consumptive coagulopathy (i.e., it is not a Kasabach-Merritt phenomenon). Platelet levels rise rapidly as the tumor regresses. RICH commonly occurs on the trunk and extremities, but also in the head and neck and the liver. RICH involutes rapidly during the first few weeks or months of life.
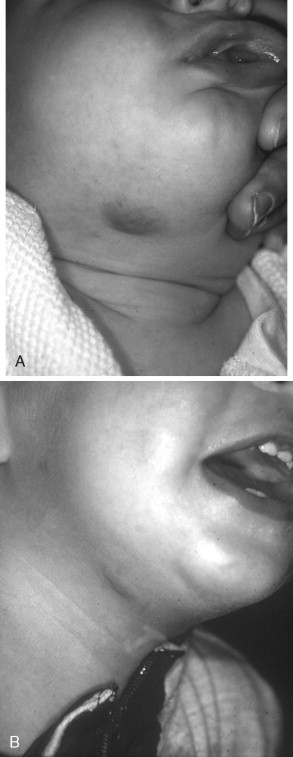
NICHs are typically ovoid, macular, or slightly raised; light gray in color with prominent coarse telangiectasias; and warm on palpation ( Figure 29-4 ). There is a predisposition for the mandibular border. An NICH is typically a wellcircumscribed lesion, averaging 5 to 6 cm in diameter. An NICH is usually not diagnosed until later in childhood because it was mistaken for common IH or presumed to be a small AVM. NICH can occur as a large lesion in the cervical and occipital areas. Sometimes lesions in this location expand during adolescence and exhibit polypoid excrescences.
RICHs and NICHs have overlapping radiologic and histologic features with IHs; however, curiously, neither type of congenital tumor immunostains for glucose transporter-1 protein (GLUT1). This raises the question of whether these congenital hemangiomas are variations in the hemangiomatous spectrum or entirely different tumors that arise in fetal life. There are more similarities than dissimilarities between IHs, RICHs, and NICHs. Furthermore, RICHs and NICHs can coexist with IHs.
MANAGEMENT
Observation and waiting for involution is appropriate for small hemangiomas that cause no harm or disfigurement. Spontaneous ulceration has been reported to occur in up to 20% of cutaneous hemangiomas ; this most frequently involves lesions on the lips, perineum, anogenital area, and extremities ( Figure 29-2 ). An ulcerated hemangioma can become infected or destroy a lip, nose, or eyelid. For small superficial ulcerations, treatment includes cleansing with application of hydrated petrolatum dressing or a topical antibiotic. Scarring is worse in ulcerated areas compared with nonulcerated areas following regression. There is some evidence to suggest that pulsed dye laser therapy can be effective for ulcerated hemangiomas. Nevertheless, pulsed dye laser therapy for proliferating and early involuting hemangiomas is not indicated because the laser cannot penetrate deep enough into the tumor to be effective. Laser application during proliferation of the hemangioma can cause ulceration, hypopigmentation, and scarring. Laser therapy does not alter the life cycle of the hemangioma. A pulsed dye laser is effective for photocoagulation of the residual telangiectasias that persist after involution.
Pharmacologic therapy is indicated if a hemangioma causes destruction, distortion, or obstruction—for example, a proliferating hemangioma producing subglottic narrowing and distortion of the growing cornea, resulting in astigmatic amblyopia or blockage of the visual axis, causing deprivation amblyopia and failure to develop binocular vision. Subglottic hemangioma can be life threatening. The symptoms are hoarseness and biphasic stridor, generally manifesting between 4 and 12 weeks of age. Approximately 50% of these infants have a cutaneous hemangioma in a beardlike distribution. A tracheostomy may be necessary if the lesions are large or unresponsive to pharmacologic therapy. Other alternatives include a carbon-dioxide laser (for unilateral lesions) and open resection.
Intralesional steroids are useful for small lesions on the face (less than 2 cm in diameter). Systemic steroids, vincristine, and interferon-α should be used only in infants with a hemangioma that (1) is rapidly growing and seriously distorting facial features; (2) causing recurrent bleeding, ulceration, or infection; and (3) impinging on critical anatomic structures. Endangering and life-threatening complications, such as high-output cardiac failure with a large tumor or multiple intrahepatic tumors, occur in 10% of hemangiomas. The responsiveness to corticosteroids, the first-line treatment, is as high as 85%, with stabilization of growth or accelerated regression. Indications for second-line therapy (vincristine, interferon-α) are failure of response to corticosteroids, contraindications to prolonged systemic corticosteroids, and complications of corticosteroids.
Resection is a consideration in all three phases of the hemangioma life cycle. Indications for excision in infancy include: obstruction of a vital structure, recurrent bleeding, and ulceration. Resection during early involution can be carried out if excision is inevitable because of postulceration scarring, expanded skin, or high possibility of fibrofatty residuum and if the scar is easily concealed or if staged excision will be necessary. Indications for resection during late childhood include damaged skin, excess skin, abnormal contour (fibrofatty residuum), distortion, and loss of tissue. Resection of a facial hemangioma, in any of the phases of its life cycle, requires careful planning. The conventional method is excision in a lenticular format with linear closure in the axis of the relaxed cutaneous tension lines, either as a single or staged procedure. This technique is applicable in some locations, such as the lips, eyelids, neck, and sometimes the scalp. Most hemangiomas are spherical, and lenticular excision (to minimize “dog ears”) causes central tension that flattens a convex area, such as the cheek or forehead, or may cause distortion of nearby structures. Hemangiomas act as a tissue expander; therefore circular excision and purse-string closure should always be the first consideration. This technique converts a circular lesion into a small circular or ellipsoid scar. Thereafter, a second excision can be either circular or lenticular. The result is the smallest possible scar with minimal distortion of surrounding structures.
RICH often leaves behind loose skin and prominent draining veins. Resection may be indicated in an end-stage RICH. NICH can be resected, and diagnosis is established; usually the indication is cosmetic.
VASCULAR MALFORMATIONS
Vascular malformations are localized abnormally formed lymphatic or blood vessels. Present at birth, some vascular malformations are obvious, whereas others manifest later in life. They grow commensurately with the patient. Malformations are divided by the preponderant vessel type and the rheologic characteristics (i.e., flow (slow- or fast-flow). The malformation may be a single vessel type or a combination of anomalous channels, often with soft tissue and skeletal overgrowth in an extremity. Combined vascular anomalies in the head and neck region are lymphaticovenous (LVM) and arteriovenous (AVM).
US is helpful in distinguishing lymphatic, venous, and arteriovenous malformations. MRI is the gold standard to determine the type and extent of the lesion; gadolinium is given as a contrast agent for enhancement.
SLOW-FLOW VASCULAR MALFORMATIONS
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


