Fig. 11.1
Clinical example of a dental implant (maxillary left central incisor) exhibiting biological complications following restoration
This chapter will illuminate the potential role of residual excess cement as an etiologic factor for the development of biological complications around dental implants. The importance of a thorough clinical examination and diagnostic radiographs will be demonstrated, and potential treatment options will be discussed.
Clinical Examination
Periodic post-treatment examination of dental implants is of utmost importance since implant complications can often be treated successfully when detected early. Without periodic re-examinations, peri-implant disease might not be detected early enough due to the absence of tangible clinical symptoms. The American Academy of Periodontology issued a paper in 2003 on periodontal maintenance stating, “patients should be evaluated at regular intervals to monitor their peri-implant status, the condition of the implant supported prostheses, and plaque control.” Some authorities recommend regular peri-implant re-evaluations every 3 months during the first year after restoration, followed by less frequent office visits thereafter. Evaluation of the dental implant includes but is not necessarily limited to radiographic examination, implant stability tests, analysis of microbial profiles, peri-implant probing, and assessment of clinical attachment levels.
Diagnostic periapical and vertical bitewing radiographs should be taken at the time of implant placement to establish baseline bone levels and at the time of delivery of the final implant-supported restoration. Subsequent radiographs should be ordered as indicated and compared to the baseline to rule out progressive peri-implant bone loss (<0.1 mm bone remodeling per year, 1 year after implant placement).
Implant stability measurements using impact resistance (Periotest, Avtec Dental, Mount Pleasant, SC, USA) or resonance frequency analysis (RFA) could be implemented during posttreatment. Automated implant stability meters are readily available that measure the implant stability quotient (ISQ) value as an indicator for the level of osseointegration in dental implants. The ISQ scale ranges from 1 to 100, and values from 55 to 85 indicate acceptable stability ranges. The cause of any implant mobility needs to be assessed carefully to distinguish between peri-implant tissue destruction due to loss of osseointegration and peri-implant mucositis due to failing (mobile or fractured) prosthetic components (Fig. 11.2a, b). However, significant amounts of peri-implant bone loss may not be detected with either of these methods due to their low sensitivity. Both methods might, however, be helpful in determining initial implant stability at the time of implant placement to assist in selecting the correct loading protocol.
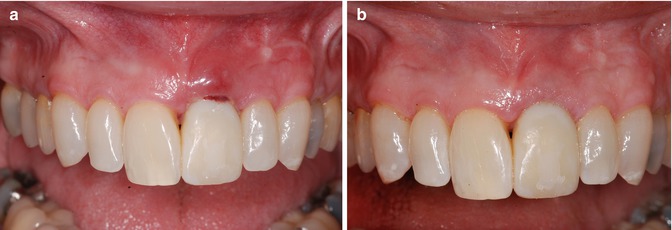

Fig. 11.2
(a) Peri-implant mucositis as a result of micro-movement due to inadequately tightened abutment screw (maxillary left central incisor). (b) Uneventful healing 2 weeks following adequate torque of abutment screw
Periodontal probing around dental implants provides important diagnostic information to evaluate peri-implant health and assist in long-term monitoring. Periodontal probing around dental implants does not seem to have a long-term effect on the soft tissue seal, since complete epithelial reattachment will occur within 5 days following probing with no signs of residual soft tissue damage. Traditionally the use of plastic periodontal probes has been recommended even though conventional metal probes do not appear to elicit any adverse effects on the implant surface or surrounding tissues. When considering probing as a test method, the difference between peri-implant probing and periodontal probing around healthy teeth must be understood. Specifically, the peri-implant probing depth typically measures the thickness of the surrounding sulcus, junctional epithelium, and connective tissue attachment and correlates, therefore, more closely with the level of the surrounding bone than the apical termination of the junctional epithelium (aJE) around dental implants. With probing around healthy teeth, the probing will generally be limited by the connective tissue fiber bundles that insert into the cementum lining the tooth root (See Chapter 1, Figure 1.5 a,b). Dental implants placed at bone level might therefore exhibit probing depths slightly greater than 4 mm at baseline (delivery of final implant-supported restoration). Increases in clinical probing depth over time, however, are usually associated with progressive loss of clinical attachment including peri-implant bone and should therefore be viewed as a sign of peri-implant disease.
It is generally believed that periodontal pathogens that cause periodontitis are also related to the onset and progression of peri-implant disease. Several microbiological tests are commercially available to measure the levels of putative periodontal pathogens either in saliva samples or through paper-point sampling from peri-implant pockets. It might, therefore, be prudent to measure spirochetes and Gram-negative mobile anaerobic bacteria levels in patients with signs of peri-implantitis to better assist in selecting appropriate treatment options.
Etiology of Biological Complications Due to Residual Excess Cement
As discussed previously in this book, bacterial colonization, foreign body reaction, corrosive effects, and allergic reactions might play a role in the etiopathogenesis of biological complications due to residual excess cement. Different luting cements exhibit varying degrees of bacterial protection against periodontal pathogens due to their inherent antibacterial activities and solubility patterns. It is also known that some luting cements might elicit significant inflammatory responses and cytotoxicity in conjunction with foreign body reactions presenting as multinucleated giant cells. Additionally, micro-movement of loose cement particles might play a role in causing biological complications around dental implants similar to mobile prosthetic components (seen in Fig. 11.2a, b) that cause peri-implant mucositis if not detected early.
Treatment Modalities
Implant success is defined as implants with no pain, mobility, or radiolucencies and no more than 0.2 mm of peri-implant bone loss annually following the first year of loading. Additionally, peri-implant hard and soft tissues should remain healthy, and the patient should be satisfied with the final result both from esthetic and functional point of view. Biological complications are one of many etiologic factors for implant failures and involve pathologic changes in the peri-implant hard and soft tissues. Inflammatory changes in response to residual excess cement (REC) are prevalent and present a therapeutic challenge in maintaining healthy peri-implant tissues. The following paragraphs will discuss several treatment modalities that will aim at removing the foreign etiologic agent (REC) and help in preserving or restoring lost peri-implant soft and hard tissue structures utilizing an incremental therapeutic approach.
While biological complications associated with residual excess cement can be due to bacterial colonization, foreign body, corrosion effects, and/or allergic reactions, removing contaminants from the implant surface and surrounding tissues is considered the most important step during surgical management.
Decontamination of the Implant Surface
Many different decontamination techniques including mechanical, chemical, and electrochemical disinfection have been studied in the past. Ultrasonic scalers, plastic-tip scalers, titanium curettes, air-powder systems, rubber cups, titanium brushes, and cotton pellets have been used in combination with various chemicals including chlorhexidine solution or gel, stannous fluoride, tetracycline, minocycline, citric acid, hydrogen peroxide, and saline to decontaminate the implant surface. Photodynamic therapy, as well as different types of laser, including Er:YAG, Nd:YAG, and CO2 lasers, have been tested in animals and clinical settings. More recently, electrochemical disinfection of dental implants using electrolysis to remove adherent bacteria from the implant surface is showing promising results as a method to decontaminate dental implants. While most of these decontamination methods have shown efficacy at removing biofilms, attempts to compare different decontamination methods have failed to show significant differences in treatment outcome.
Removal of residual excess cement requires mechanical debridement of the implant surface with either hand- or power-driven devices and depends significantly on the morphology of the peri-implant defect. A recent study indicates that implants surrounded by bony walls are less accessible for mechanical debridement even when air-flow devices are used. Clinicians currently use glycine-based air-flow devices as well as chemicals, including chlorhexidine and tetracycline solutions, to decontaminate the implant surface. It is still not clear if decontaminating implant surfaces will result in re-osseointegration of the entire implant. Even pristine implants placed into artificially created peri-implant defects show significantly less bone to implant contact as the width of the gap increased. Although some animal studies have shown the possibility of re-osseointegration of previously contaminated implant surfaces, achievement of re-osseointegration in a clinical setting might still be elusive. The therapeutic goal is the preparation of an implant surface that is biologically compatible with the peri-implant tissues and no signs of inflammation such as swelling, bleeding, or suppuration.
Nonsurgical Approach to Remove REC
“Less is more,” a phrase coined by Robert Browning (1855), still holds true for many procedures in clinical dentistry today. Generally, provided the goal of therapy is achieved, the less invasive the intervention, the more postoperative comfort for the patient, and the faster the healing occurs. The less the mucoperiosteal flaps need to be elevated, the more the mucogingival architecture will be preserved. The following case history (Fig. 11.3a–g) demonstrates a clinical example of residual excess cement causing peri-implant mucositis that was subsequently treated utilizing the least invasive approach possible. The immediate implant was restored 12 weeks following implantation with a zirconia computer-aided design-computer-aided manufactured (CAD-CAM) abutment, utilizing temporary luting cement (TempBond, Kerr). The patient presented two years following restoration with signs of peri-implant mucositis in combination with tenderness to palpation of the peri-implant soft tissues (Fig. 11.3a). A typical “peripheral eggshell effect” was evident upon radiographic examination, confirming the diagnosis of REC (Fig. 11.3b). Additionally, no radiographic bone loss could be detected ruling out the diagnosis of peri-implantitis. The treatment consisted of meticulous cement removal utilizing hand instruments and piezoelectric devices followed by copious irrigation with chlorhexidine gluconate solution and digital pressure to achieve adequate hemostasis postoperatively (Fig. 11.3c, d).
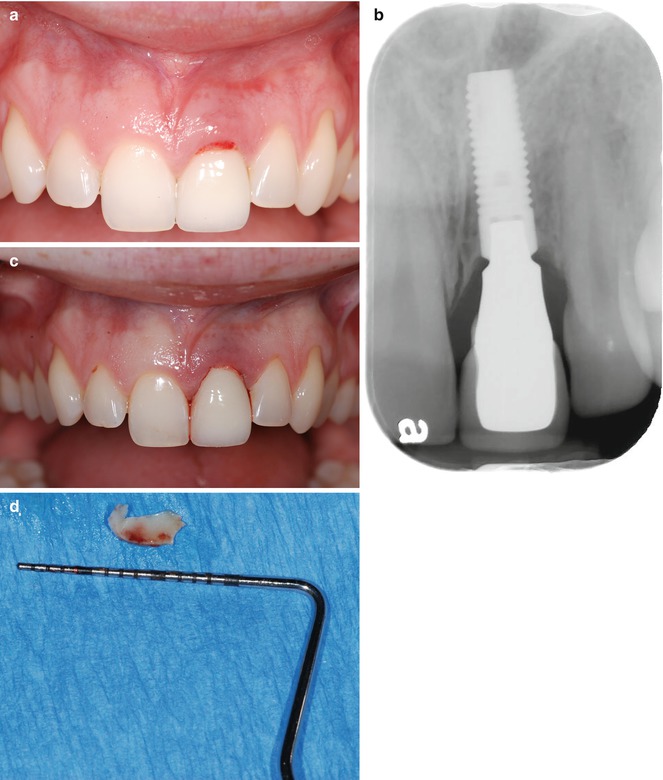
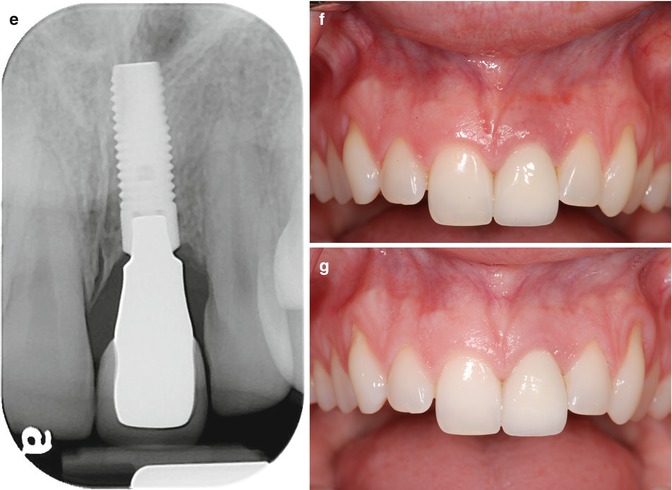


Fig. 11.3
(a) Clinical example of residual excess cement causing peri-implant mucositis (maxillary left central incisor). (b) Radiograph with typical “peripheral eggshell” effect indicating residual excess cement at crown/abutment margin. (c) Status post removal of residual excess cement; (d) excess cement removed. (e) Radiographic evaluation following cement removal indicating lack of REC; (f) 2 weeks later. (g) Uneventful healing 4 months following removal of REC
Uneventful healing was evident at 2 weeks, and complete resolution of the soft tissue defect was observed 4 months following therapeutic intervention (Fig. 11.3e–g). Subgingival debridement, including removal of excess luting cement, traditionally entails the use of plastic curettes and polishing pastes. Most plastic instruments, however, are highly flexible and can therefore not be used to dislodge subgingival calculus and dental cements with high bond strengths. Additionally, those instruments carry an increased risk of leaving remnants of the instrument material in the surgical site, compromising the wound healing. Stainless steel hand instruments, on the other hand, might leave significant damage on the treated implant surface, with subsequent increased plaque accumulation and biofilm growth. Titanium instruments are therefore considered state of the art to avoid the mentioned shortcomings, yet establishing a biocompatible implant surface after mechanical debridement.
Magnetostrictive or piezoelectric devices also seem to damage the implant surface if conventional tips are used. Copper alloy or plastic-covered tips are believed to minimize the damaging effect on the implant surface but may also increase the risk of leaving material remnants behind. Irrespective of the instrument used, it seems to be a “conditio sine qua non” to remove the REC as thoroughly as possible to allow for soft tissue healing and “restitutio ad integrum.” This should be accomplished even at the potential expense of damaging the implant surface, if necessary, since unequivocal evidence is missing to support the notion that a damaged implant surface will eventually lead to peri-implant mucositis or peri-implantitis.
Another, slightly more invasive, approach consists of removing the entire cemented implant-supported restoration to obtain extraoral access for cement removal; this treatment modality is mainly indicated in the esthetic zone to avoid negative esthetic sequelae following surgical intervention.
Case Report (Fig. 11.4a–e)
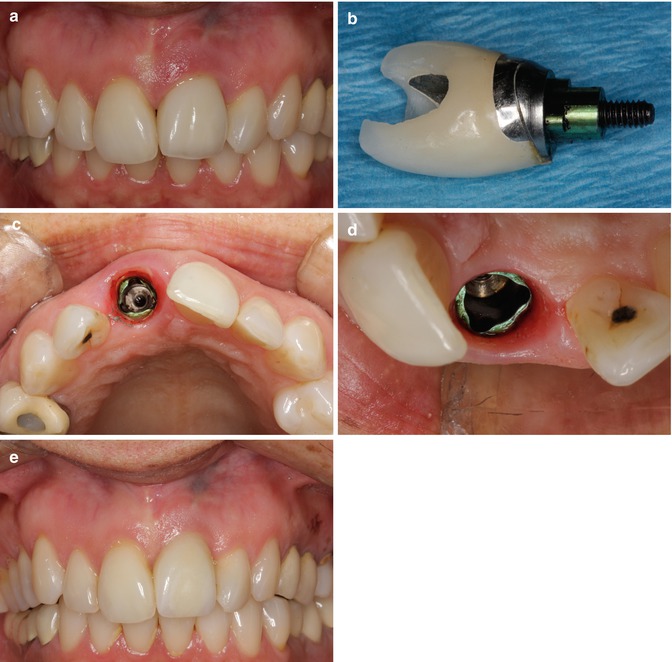
Fig. 11.4
(a) Inflammation of the peri-implant mucosa. Residual cement was not detected on radiographic survey. However, considering the restorative design with a deep cement margin, incomplete removal of cement was suspected. (b) The decision was made to remove the crown and the abutment, by creating an access to the abutment screw. Upon removal of the crown/abutment complex, residual cement was found. Seen here in the distal- buccal aspect of the sulcus. (c) Occlusal view immediately after removal of the crown. Inflammation is found at the sulcus. (d) Six weeks after removal of cement. Upon removal of the crown/abutment complex, absence of inflammation was found. (e) Facial view 10 months after the initial visit. The crown and abutment were remade
A female patient 45 years of age had an implant placed several years earlier. She presented complaining of inflammation around the implant site. A radiograph did not indicate REC presence; however, this is not uncommon as described in the previous chapters within this book. REC was suspected due to the depth of the restorative margin, so a procedure designed to evaluate the site was proposed to and accepted by the patient. Initially the crown/abutment complex would have to be removed. To obtain access to the contaminated abutment, a hole was prepared through the implant-supported crown to access the implant-abutment screw. The crown and abutment were removed by counter-torquing the abutment screw (Fig. 11.4b, c). Residual excess cement was present as suspected. The foreign material was carefully removed, and the site cleaned with chlorhexidine gluconate solution. The crown/abutment complex was also cleaned then retightened to the recommended torque and the screw access site within the crown restored with composite, thereby converting a cement-retained restoration into a screw-retained restoration. Significant resolution of the peri-implant mucositis was observed 6 weeks following treatment (Fig. 11.4d). Subsequently, a more appropriately designed abutment with a more coronally placed cement margin and a new crown were made and delivered. Ten months after the initial visit, the implant restoration remained clinically inflammation free (Fig. 11.4e) with complete resolution.
Surgical Approach to Remove Residual Excess Cement (REC)
The advantage of a noninvasive approach to remove REC is evident, especially in situations that are esthetically challenging, and adequate access for cement removal is likely, for example the maxillary anterior zone. In situations, however, where complete cement removal cannot be accomplished utilizing a closed-flap approach, surgical intervention becomes more appropriate. Raising soft tissue flaps to allow access to the site may also be combined with antimicrobial therapy, regenerative techniques, or adjunctive laser therapy. Along with an improvement to access the implant body for debridement, the soft tissues may also be surgically accessed allowing removal of any foreign body matter that may also be present.
Most of the surgical techniques employed to treat peri-implantitis as a result of REC have been derived from and used successfully to treat periodontal lesions around natural teeth. Access flap, removal of granulation and/or granulomatous tissue, and surface decontamination are a common practice in treating periodontal or peri-implant defects.
A clinical example demonstrating the effectiveness of open flap debridement is provided in the following case example: A 72-year-old male in good health had 2 implants placed in a one-stage surgical procedure, with healing caps, in the maxillary first and second premolar sites. After allowing for a healing period of 4 months, the implants were deemed sufficiently osseointegrated to allow for final restoration and the treatment completed. After restoration, the first annual recall appointment revealed no issues. However at the second annual patient follow-up examination, intraoral radiographs revealed REC on both implants (Fig. 11.5a, b
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


