Traditional methods of treatment of immature root with necrotic pulp and apical periodontitis pose multiple challenges. These challenges include disinfection of the root canal with standard protocols that aggressively use endodontic files, filling the root canal with an open apex that provides no barrier for stopping the root filling material before impinging on the periodontal tissues, and the susceptibility of the teeth to fracture because of their thin roots. Disinfection using sodium hypochlorite, apical barrier formation using calcium hydroxide as well as mineral trioxide aggregate, and pulp revascularization of fractured tooth with the help of blood clot and collagen-enhanced matrix has been discussed in detail in this article.
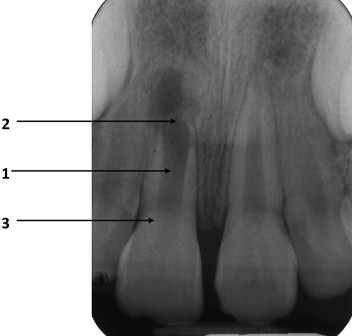
The immature root with a necrotic pulp and apical periodontitis ( Fig. 1 ) presents multiple challenges to successful treatment.
- 1.
The infected root canal space cannot be disinfected with the standard root canal protocol with the aggressive use of endodontic files.
- 2.
Once the microbial phase of the treatment is complete, filling the root canal is difficult because the open apex provides no barrier for stopping the root filling material before impinging on the periodontal tissues.
- 3.
Even when the challenges described earlier are overcome, the roots of these teeth are thin with a higher susceptibility to fracture.
These problems are overcome by using a disinfection protocol that does not include root canal instrumentation, stimulating the formation of a hard tissue barrier or providing an artificial apical barrier to allow for optimal filling of the canal, and reinforcing the weakened root against fracture during and after an apical stop is provided.
Traditional technique
Disinfection of the Canal
Because in most cases nonvital teeth are infected, the first phase of treatment is to disinfect the root canal system to ensure periapical healing. The canal length is estimated with a parallel preoperative radiograph, and after access to the canal is made, a file is placed to this length. After the length has been confirmed radiographically, depending on the thickness of the remaining dentinal walls either a very light filing or no filing is performed with copious irrigation with 0.5% sodium hypochlorite. A lower strength of sodium hypochlorite is used because of the danger of placing it through the apex of immature teeth. The lower strength of sodium hypochlorite is compensated by the volume of the irrigant used. An irrigation needle that can passively reach close to the apical length is useful in disinfecting the canals of immature teeth. The intra-canal medication is placed when the irrigant leaving the canal is clean of debris. Newer irrigation protocols such as EndoVac (Discus Dental, Culver City, CA, USA) or use of ultrasound may be useful in immature canals.
The canal is dried with paper points, and a creamy mix of calcium hydroxide is spun into the canal with a lentulospiral instrument. The disinfecting action of calcium hydroxide (in addition to instrumentation and irrigation) is effective after its application for at least 1 week, so that the continuation of treatment can take place any time after 1 week. Further treatment should not be delayed for more than 1 month because the calcium hydroxide could be washed out by tissue fluids through the open apex, leaving the canal susceptible to reinfection.
A new disinfection medicament has been used when revascularization is attempted (discussed in later section). This medicament has been extensively studied by Sato and colleagues and Hoshino and colleagues. It comprises metronidazole, ciprofloxacin, and minocycline in a saline or glycerin vehicle. A recent study by Windley and colleagues showed the effectiveness of the tri-antibiotic paste when used for a month on immature infected dog teeth that had been irrigated with only sodium hypochlorite.
Hard Tissue Apical Barrier
Traditional method
The formation of the hard tissue barrier at the apex requires an environment similar to that required for hard tissue formation in vital pulp therapy, that is a mild inflammatory stimulus to initiate healing and a bacteria-free environment to ensure that the inflammation is not progressive.
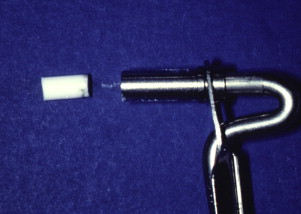
As with vital pulp therapy, calcium hydroxide is used for this procedure. Pure calcium hydroxide powder is mixed with sterile saline (or anesthetic solution) to a thick (powdery) consistency ( Fig. 2 ). Ready mixed commercially available calcium hydroxide can also be used. The calcium hydroxide is packed against the apical soft tissue with a plugger or a thick point to initiate hard tissue formation. This step is followed by backfilling with calcium hydroxide to completely fill the canal thus ensuring a bacteria-free canal with little chance of reinfection during the 6 to 18 months required for the hard tissue formation at the apex. The calcium hydroxide is meticulously removed from the access cavity to the level of the root orifices, and a well-sealing temporary filling is placed. When a radiograph is taken, the canal should seem to have become calcified, indicating that the entire canal has been filled with the calcium hydroxide ( Fig. 3 ). Because calcium hydroxide washout is evaluated by its relative radiodensity in the canal, it is prudent to use a calcium hydroxide mixture without the addition of a radiopaque substance such as barium sulfate. These additives do not wash out as readily as calcium hydroxide, so if they are present in the canal, evaluation of washout is not possible.
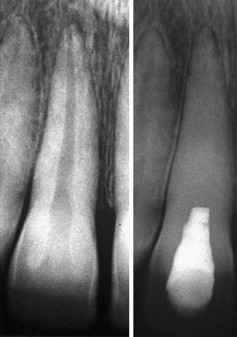
At 3 months’ interval a radiograph is taken to evaluate if a hard tissue barrier has formed and if the calcium hydroxide has washed out of the canal. This is assessed to have occurred if the canal can be seen again radiographically. If calcium hydroxide washout is seen, it is replaced as before. If no washout is evident, it can be left intact for another 3 months. Excessive calcium hydroxide dressing changes should be avoided if possible because the initial toxicity of the material is believed to delay healing.
When completion of a hard tissue barrier is suspected, the calcium hydroxide is washed out of the canal with sodium hypochlorite and a radiograph is taken to evaluate the radiodensity of the apical stop. A file that can easily reach the apex is used to gently probe for a stop at the apex. The canal is filled after the presence of a hard tissue barrier is indicated radiographically and the barrier is probed with an instrument.
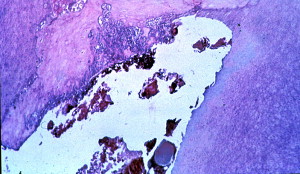
The hard tissue barrier that forms has been described as “Swiss cheese–like” ( Fig. 4 ) because many soft tissue inclusions are still present inside the hard tissue during the time a barrier that can resist a filling material is formed. The soft filling material therefore often passes through the apex in the form of a sealer or filling material puff. The hard tissue barrier is formed at the site of healing of the periodontal granulation tissue. This site does not always conform to the radiographic apex of the tooth. Therefore when the presence of the hard tissue is felt with a point or file, it may be short of the radiographic apex of the tooth. It is important not to force the file to the radiographic apex so as to avoid destruction of the formed barrier.
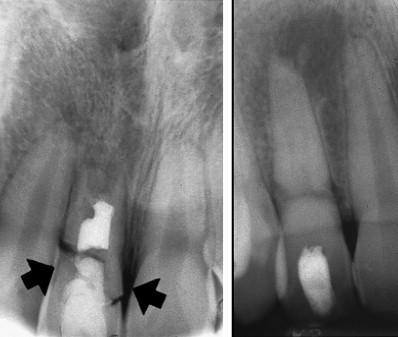
The traditional calcium hydroxide apexification technique has been extensively studied and is proved to have a high success rate. However, the technique has some disadvantages. The primary disadvantage is that it typically takes between 6 and 18 months for the body to form the hard tissue barrier. The patient needs to report every 3 months to evaluate whether the calcium hydroxide has washed out and/or the barrier is complete enough to provide a stop to a filling material. This requires patient compliance for up to 6 visits before the procedure is completed. It has also been shown that the use of calcium hydroxide weakens the resistance of the dentin to fracture. Thus it is common for the patient to sustain another injury and also fracture the root before the hard tissue barrier is formed ( Fig. 5 ).
Mineral trioxide aggregate barrier
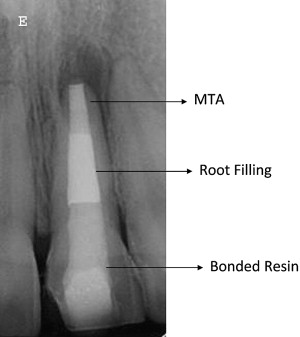
Mineral trioxide aggregate (MTA) is used to create a hard tissue barrier after the disinfection of the canal ( Fig. 6 ). Calcium sulfate (or similar material) is pushed through the apex to provide a resorbable extraradicular barrier against which the MTA is packed. The MTA is mixed and placed into the apical 3 to 4 mm of the canal in a manner similar to the placement of calcium hydroxide. A wet cotton pellet can be placed against the MTA and left for at least 6 hours and then the entire canal filled with a root filling material or the filling can be placed immediately because the tissue fluids of the open apex may provide enough moisture to ensure that the MTA sets sufficiently. The cervical canal is then reinforced with composite resin to below the level of the marginal bone as described later in the article (see Fig. 6 ).
Several case reports have been published using this MTA apical barrier technique, and it has steadily gained popularity with clinicians. At present, there is no prospective long-term outcome study that compares the success rate of this technique with that of the traditional calcium hydroxide technique.
Because the apical diameter is larger than the coronal diameter of most of the canals, a softened filling technique is indicated for these teeth. Care must be taken to avoid excessive lateral force during filling because of the thin walls of the root.
The apexification procedure has become a predictably successful procedure. However, the thin dentinal walls still present a clinical problem. Should secondary injuries occur, teeth with thin dentinal walls are more susceptible to fractures that render them nonrestorable. It has been reported that approximately 30% of these teeth will fracture during or after endodontic treatment (see Fig. 5 ). Some clinicians have therefore questioned the advisability of the apexification procedure and have opted for more radical treatment procedures, including extraction followed by extensive restorative procedures such as dental implants. Studies have shown that intracoronal bonded restorations can internally strengthen endodontically treated teeth and increase their resistance to fracture. Thus after root filling, the material should be removed to below the level of marginal bone and a bonded resin filling placed (see Fig. 6 ).
Routine recall evaluation should be performed to determine the success in the prevention or treatment of apical periodontitis. Restorative procedures should be assessed to ensure that they do not promote root fractures.
Periapical healing and the formation of a hard tissue barrier occurs predictably with long-term calcium hydroxide treatment (79%–96%). However, long-term survival is jeopardized by the fracture potential of the thin dentinal walls of these teeth. It is expected that the newer techniques of internally strengthening the teeth described earlier will increase their long-term survivability.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


