With the appearance of more in vivo and ex vivo publications, methacrylate based resin sealers are becoming more popular in endodontics. Their ease of use and favorable clinical performance offer an attractive alternative to conventional endodontics. This article reviews the development of resin-based sealers and biocompatibility tests. The many, mostly opposing views are analyzed to put what has been published thus far in perspective. A critical analysis of the facts leads to the consensus that methacrylate based resin sealers are here to stay and offer a suitable alternative to conventional endodontic treatment.
Development leading to resin sealers
The concept of resin bonding in dentistry was introduced in the mid-1950s by Buonocore, who advocated the use of an acid to demineralize enamel. Skepticism slowly gave way to general acceptance. However, bonding materials and techniques have completely changed over the course of 50 years. During the initial development only hydrophobic resins were available; these have been replaced by hydrophilic resins over time. Furthermore, about 30 years of research resulted in a change from using 85% phosphoric acid liquid for 60 seconds to etch enamel to 35% phosphoric acid gels for 15 seconds to etch dentin and enamel. Although early attempts were strictly focused on preventive and restorative dentistry, it was only a matter of time before orthodontics and then endodontics embraced this concept. Usually, when new materials and techniques are introduced, there is an initial reluctance on the part of practitioners to abandon trusted and proven methods until evidence that is sufficiently convincing to change established techniques is generated.
The objective of this article is to provide information about methacrylate-based resin sealers (MBRS) on which practitioners can base their decision to consider changing established techniques and embrace a new one. This decision cannot be made by presenting empiric data, but by offering an analysis of scientific evidence from ex vivo and in vivo research. Based on their successful long-track record, gutta-percha and zinc oxide, eugenol, and other conventional sealers, have served as the gold standard for comparison.
One of the factors that was instrumental in the development of resin-based sealers was the recognition that gutta-percha does not bond to dentin or to any conventionally used sealer, such as zinc oxide-eugenol (ZOE)-based cements and epoxy resins such as AH-26 or AH Plus. Although these materials are being used successfully, an ideal root canal sealer should be capable of bonding to root canal dentin and to gutta-percha, thus preventing microleakage. Recent advances in adhesive technology have led to the introduction of a new generation of endodontic sealers and filling materials, that are based on adhesive properties and polymer resin technology. These materials are capable of forming a hybrid layer and penetrating deep into dentinal tubules by virtue of their hydrophilic properties.
Early attempts at using resins were reported in 1978 by Tidmarsh, who suggested that a low-viscosity resin could have the potential to be used in root canal obturation. Of the bonding agents that were used in restorative dentistry, the early generations did not use an acid to remove the smear layer and therefore bonded to it. This resulted in a weak bond and did not prevent bacterial leakage. Later generations that used 35% phosphoric acid gels for the removal of the smear layer were more promising. Furthermore, the early resins were hydrophobic and therefore their interaction was adversely affected by moisture in the dentin. The latest bonding agents are hydrophilic and they derive their adhesive properties from micromechanical interlocking by penetrating into dentinal tubules, thus creating an attachment mechanism along with an intimate hybrid layer when they come in direct contact with the surrounding collagen fibrillar intertubular network. The latter requires careful treatment and it has been shown that the collagen network of dentin can be best preserved using 17% to 19% EDTA or low concentrations of citric acid solution as the final rinse. Effective removal of the smear layer before filling the canals will enhance the ability of these bonding agents to enter the dentinal tubules and improve the sealing of the root canal system by increasing the contact surface area. The presence of organic debris along with bacteria within the matrix of the smear layer represents an undesirable interface between filling material and dentin. Furthermore, the sequence of the irrigating solutions has been shown to be a factor. A 5% sodium hypochlorite (NaOCl) solution followed by 17% EDTA or 50% citric acid seems to be the most effective combination.
Zidan and El Deeb were among the first to attempt to establish adhesion to dentin walls in vitro with the use of Scotchbond (3M ESPE, St Paul, MN, USA). Apical microleakage with gutta-percha and the bonding agent was significantly less than in root canals obturated with gutta-percha and Tubli-Seal (SybronEndo, West Collins, Orange, CA, USA), a ZOE-based root canal sealer. Handling properties, radiopacity, and the difficulty of removing the sealer in case of retreatment were some of the drawbacks that were experienced. Other possible bonding systems have subsequently been reported in the literature. Leonard and colleagues compared the effectiveness of a combination of the dentin bonding agent 4-methacryloyloxyethy trimellitate anhydride (4-META) and the resin C&B Metabond (Parkell Inc, Edgewood, NY, USA), which was commercialized a few years later as MetaSEAL (Parkell Inc, Edgewood, NY, USA), and the glass ionomer cement Ketac-Endo (3M ESPE, St Paul, MN, USA) for sealing of the root canal system. The coronal and apical seals were tested by means of dye penetration, and both materials showed some evidence of dye leakage. However, the sealing ability of the bonding agent and resin was significantly better. This was further supported by scanning electron microscopy (SEM) of the interface sealer and dentin, indicating the presence of a hybrid layer and resin tags penetrating into the dentinal tubules. Despite these positive features, the materials seemed to be technique sensitive. Nikaido and colleagues, Morris and colleagues, and Erdemir and colleagues, showed that the use of sodium hypochlorite and hydrogen peroxide or a combination of both irrigants, decreased the bond strength to dentin by adversely affecting the tensile bond strength to bovine dentin. Hydrogen peroxide breaks down to water and oxygen, whereas the combination of sodium hypochlorite and hydrogen peroxide allows for the formation of oxygen, which inhibits polymerization of the adhesive materials. However, irrigation with chlorhexidine did not exhibit these adverse effects.
ALL-BOND 2 adhesive (Bisco, Itasca, IL) and Scotchbond Multi-purpose Plus adhesive in combination with gutta-percha and an epoxy resin–based root canal sealer AH-26 (Dentsply-Maillefer, Switzerland) was also tested for leakage with a 2% methylene blue solution. It was reported that root canals that had the combination of bonding agents with gutta-percha and the epoxy resin sealer leaked significantly less than the controls in which the root canals were obturated with gutta-percha and AH-26. Although no problems were experienced with respect to the working time of the bonding agents, the complexity of the technique (it required many steps) made the use of bonding agents impractical for root canal obturation. Of additional concern is the use of bonding agents containing 2-hydroxyethyl methacrylate (HEMA), which, when extruded beyond the apex into bone, could sensitize patients, particularly if they are from Nordic countries or have genetic make-up that originates there.
Ahlberg and Tay tested a methacrylate-based bone cement normally used in orthopedic surgery, in which the monomer from N -butyl methacrylate was changed to tetrahydrofurfuryl methacrylate with 1% N’N’ -dimethyl p -toluidine as the activator. The powder consisted of poly(ethyl methacrylate) with a molecular weight of 150,000 to 1,500,000 and a particle size of 15 to 100 μm. They used this formulation to obturate in vitro root canals of human teeth with gutta-percha cones; the control canals were filled with gutta-percha only. The root canals filled with the resin and gutta-percha leaked significantly less than the controls. Scanning electron microscope observation of the interface revealed a bond not only between the resin-based sealer and the root canal walls but also between the sealer and gutta-percha. With respect to their handling properties, the material was found to be easy to place in the root canal and the working time was approximately 50 minutes. The investigators postulated that, because the smear layer was not effectively removed, bonding to the root canal walls may be attributed to the low viscosity of the resin itself, whereas the ability to bond to gutta-percha was attributed to dissolution of the gutta-percha surface.
Kataoka and colleagues analyzed the coronal and apical sealing properties of a newly developed resin-based root canal sealer composed of vinylidine fluoride/hexafluoropropylene copolymer, methyl methacrylate, zirconia, and tributylborane as the catalyst, used in conjunction with gutta-percha cones in root canals, which were pretreated with dentin conditioners and primers. They also analyzed the tensile bond strength and used SEM to analyze the interfaces. The test material revealed a significantly higher sealing ability than Pulp Canal Sealer EWT (Sybron Kerr, Romulus, MI, USA) and Sealapex (Sybron Kerr, Romulus, MI, USA), which were used as controls. When the canal walls were pretreated with EDTA and further application of glutaraldehyde/2-hydroxyethyl methacrylate primers, higher bond strength values were recorded. SEM observation revealed the presence of a hybrid layer approximately 2 μm thick, formed by the penetration of the resin into the dentin with only a few gaps at the interface between the sealer and the root canal walls. Based on these observations, the investigators suggested that the tested resin-based sealer had many useful properties for root canal obturation, such as adhesiveness to dentin and gutta-percha while exhibiting good sealing properties.
According to the above information these experimental formulations have the potential to bond to the root canal walls provided the smear layer is removed.
Methacrylate-based resin sealers
MBRS are new in endodontics and are derived from polymer chemistry technology initially developed for adhesive restorative dentistry, albeit in modified formulations and viscosities as determined by the specific demands in endodontics. This article focuses on 2 systems as they dominate the market:
- 1.
EndoREZ (Ultradent Products Inc, South Jordan, UT, USA) and
- 2.
RealSeal (Sybron Dental Specialties, Orange, CA, USA).
Pentron Clinical Technologies (Wallingford, CT, USA) was recently acquired by Sybron Dental Specialties, which includes the Resilon-Epiphany system, now marketed as RealSeal. Therefore products such as SimpliFill (LightSpeed Technology Inc, San Antonio, TX, USA), InnoEndo (Heraeus Kulzer, Armonk, NJ, USA), and Resinate (Obtura Spartan, Fenton, MO, USA) and Resilon-Epiphany are now all categorized under the name RealSeal.
Methacrylate-based resin sealers
MBRS are new in endodontics and are derived from polymer chemistry technology initially developed for adhesive restorative dentistry, albeit in modified formulations and viscosities as determined by the specific demands in endodontics. This article focuses on 2 systems as they dominate the market:
- 1.
EndoREZ (Ultradent Products Inc, South Jordan, UT, USA) and
- 2.
RealSeal (Sybron Dental Specialties, Orange, CA, USA).
Pentron Clinical Technologies (Wallingford, CT, USA) was recently acquired by Sybron Dental Specialties, which includes the Resilon-Epiphany system, now marketed as RealSeal. Therefore products such as SimpliFill (LightSpeed Technology Inc, San Antonio, TX, USA), InnoEndo (Heraeus Kulzer, Armonk, NJ, USA), and Resinate (Obtura Spartan, Fenton, MO, USA) and Resilon-Epiphany are now all categorized under the name RealSeal.
EndoREZ
EndoREZ (ER) is a hydrophilic, two-component (base and catalysts), dual-curing self-priming sealer. The formulation can be described as follows:
-
The EndoREZ base contains:
-
a bismuth compound as the radiopaque filler
-
small amounts of other fillers
-
diurethane dimethacrylate
-
triethylene glycol dimethacrylate
-
a peroxide initiator
-
a photo initiator (not chamfer quinone).
-
The EndoREZ catalyst contains:
-
a bismuth compound as the radiopaque filler
-
small amounts of other fillers
-
diurethane dimethacrylate
-
triethylene glycol dimethacrylate.
The sealer can be used with gutta-percha or with resin-coated gutta-percha cones, the latter with the objective of establishing continuous adhesion (uniblock or monobloc) between all materials. The sealer is supplied in a double barrel auto mixing and delivery syringe and meets the basic requirements of an endodontic sealer. The manufacturer recommends that after preparation the root canal walls should remain slightly moist to take maximum advantage of the hydrophilic properties of the sealer, thus allowing for resin tags to penetrate into the dentinal tubules and the formation of a hybrid layer with the collagen fiber network. However, too much water can cause water permeation during the polymerization process and results in the entrapment of water droplets in the sealer, resulting in bond disruption and an increase in leakage. Delivery through the tiny opening and the hydraulics involved when using a NaviTip (Ultradent Products Inc, South Jordan, UT, USA) produces a sealer free from air bubbles that fills the canal with a homogeneous layer. The sealer is radiopaque and has favorable low viscosity properties. Low viscosity plays a significant role in the handling properties and makes it useful for placement in wide or narrow root canals; it provides a good adaptation to the intricacies of the dentin walls. EndoREZ bonds well to root canal walls but not to gutta-percha, which constitutes a potential weakness, as a path for bacterial leakage may exist. To address this issue and to establish a bond between sealer and dentin and between sealer and gutta-percha, resin-coated gutta-percha cones (RCGP) cones (Ultradent Products Inc, South Jordan, UT, USA) were introduced.
The combination of these materials establishes the so-called monobloc and is the reason for the superior sealing properties of the system. The objective of the EndoREZ sealer is to establish a hermetic seal, rather than high bond strength adhesion, that is, optimum softness or hardness while providing a maximum seal.
The RCGP cones can be used with an accelerator, which serves a dual purpose. The polymerization reaction of the EndoREZ is accelerated (within 4–5 minutes) allowing for immediate continuation of the restorative phase should the practitioner choose to do so and bonding of the EndoREZ to the RCGP cones is promoted, thus establishing a monobloc.
RealSeal (Resilon/Epiphany)
Resilon is composed of a polymer-based resin (polycaprolactone), bioactive glass, bismuth oxide, barium sulfate and coloring agents. The sealer is a dual-cure sealer, composed of urethane dimethacrylate (UDMA), poly dimethacrylate (PEGDMA), ethoxylated bisphenol A dimethacrylate (EBPADMA) and bisphenol A glycidyl methacrylate (BIS-GMA), barium borosilicate, barium sulfate (BaSO 4 ), bismuth oxychloride, calcium hydroxide, photo initiators, and a thinning resin. In addition the system comes with a self-etching primer. The premise behind the material is the formation of a monobloc, that is, the primer forms a hybrid layer with dentin, which bonds to sealer, and then bonds to the Resilon core. The ability of Resilon to bond to methacrylate-based root canal sealers has also been questioned because the amount of dimethacrylate in the thermoplastic composite may not be optimum for chemical coupling. However, when surface roughness was established, the micromechanical interlocking increased the mean bond strength significantly.
Biocompatibility
Several early publications (2001 and 2003) have reported on the biocompatibility and adhesiveness of EndoREZ. Since then numerous publications have appeared, testing different MBRS formulations and using a variety of techniques, which to a large extent have caused more controversy and confusion than answering the following basic questions:
- 1.
Are resin-based sealers safe?
- 2.
Can they be used successfully in patients?
- 3.
Will they ultimately replace gutta-percha and conventional sealers?
- 4.
Will they last as long as conventional materials?
- 5.
Are they easier to use than conventional materials?
Toxicology studies in vitro
One of the requirements of any dental material for use in humans is that it should be biocompatible. Numerous investigators have conducted cytotoxicity studies ex vivo using cell cultures and in vivo in laboratory animals. The results between investigators are contradictory. Huang and colleagues showed that the elution compounds from MBRS, zinc oxide-eugenol and calcium hydroxide-based sealers were cytotoxic to primary human periodontal ligament cultures and V79 cells, with calcium hydroxide being the least toxic. Huang and co-workers, reported that the highest level of DNA damage was induced by epoxy resin–based sealers, in this case Topseal (Dentsply, Konstanz, Switzerland), AH-26, and AH Plus. Koulaouzidou and colleagues reported similar results. AH-26 had a severe cytotoxic effect, whereas Topseal and AH Plus had markedly lower effects. These findings are surprising as the basic formulation of AH-26 and Topseal is the same. Bouillaguet and colleagues, reported that: “Most materials pose significant cytotoxic risks and that cytotoxicity generally decreased with time.” At 72 hours, GuttaFlow became significantly less toxic than AH Plus, Epiphany sealer, and Resilon. Other investigators, such as Key and colleagues found Epiphany to be less toxic than Grossman’s sealer. However, Epiphany was more cytotoxic than Sealapex after 1 hour, but less after 24 hours. Epiphany was more cytotoxic than conventional materials. In a more recent publication similar findings were reported. According to Lodiene and colleagues the multi-methacrylate-based resin (Epiphany) root canal sealer was significantly more toxic to L-929 cells than the silicone-based RoekoSeal and the single methacrylate-based EndoREZ root canal sealers. AH Plus showed intermediate toxicity.
Based on the these findings it seems that no sealer is universally accepted as being nontoxic. Furthermore, the investigators mentioned earlier have reported completely opposite findings, which makes selection of a sealer without drawbacks difficult, if not impossible. Therefore it is necessary to conduct a careful and critical analysis of the various ex vivo research methodologies to reach a consensus. It is also important to correlate the results of the various techniques with the clinical performance of the same material or materials. Oliver and Abbott reported that clinical and in vitro data frequently contradict each other.
Toxicology studies in vivo
The early studies on which the launch of EndoREZ was based were conducted by Louw and colleagues and Becce and Pameijer who reported that EndoREZ was mildly irritating, but within acceptable standards (1.5° is the acceptable limit). Further evidence of biocompatibility was published by Zmener and Zmener and colleagues. In other related studies (Pameijer, 2002, unpublished data), EndoREZ and Epiphany/Resilon reacted more favorably than the control AH Plus. Preoperative and postoperative radiographs were made and root canal treatment was performed according to a standardized protocol using a rubber dam in subhuman primates. Histologic observations were made at various time periods: 30 days to determine an early reaction and from 3 months to 6 months posttreatment for long-term reactions. The results can be summarized as follows. Ten EndoREZ root canal treated teeth scored a mean inflammatory reaction after 26 days of 1.5°. After 90 days, out of 21 root fills, 4 had extruded sealer with an inflammatory mean of 0.8°. Good apical adaptation scored a lower mean inflammation of 0.4°. None of the periapical areas of the roots at either time period showed bone resorption. The control sealer (AH Plus) had a mean inflammatory reaction of 1.3° after 26 days and 1.0° after 90 days. Epiphany, which was tested according to the same protocol, scored a mean inflammatory reaction of all root fills of 1.2° after 120 days (13 teeth), whereas the inflammation of bone was 0.4°. Control teeth (AH Plus) had a mean inflammatory reaction of 2°, and a bone inflammation of 1°.
Both materials clearly reacted more favorably than the control AH Plus.
These results were confirmed by Zmener. The severity of the reaction decreased over time. Zmener and colleagues conducted a histologic and histometric study in which silicone tubes filled with EndoREZ were implanted in the tibias of rats for a period of 10 days and 60 days. At the 10-day observation period, the number of inflammatory cells in contact with the sealer was significantly higher. After 60 days, the initial inflammatory reaction was resolved and newly formed healthy bone was observed surrounding the implants. Thus, after early mild irritation the material reacted in a biocompatible fashion allowing healing of bone. In contrast Sousa and colleagues tested AH Plus, EndoREZ, and Epiphany in guinea pigs over 4 and 12 weeks. They reported a severe reaction for EndoREZ; AH Plus was also severe after 4 weeks and moderate after 12 weeks. Only Epiphany showed intraosseous biocompatibility.
Examples of sealer and point biocompatibility testing
The periapical tissues can react to extrusion of a sealer and/or point in several ways:
- 1.
It can cause an inflammatory reaction
- 2.
It can be regarded as a foreign body and be encapsulated
- 3.
A sealer can be present without causing inflammatory reactions and is not encapsulated
- 4.
The sealer can be resorbed over time, with or without an inflammatory reaction.
As mentioned earlier, a material causing an inflammatory reaction is not necessarily bad and the outcome depends on the intensity and duration of the inflammatory process and the ability of the natural defense mechanisms of the body to manage the reaction. Biocompatibility should be construed in a broader sense. If over a reasonable period of time (30–60 days) healing occurs after an initial irritating reaction, a material can still be considered biocompatible. None of the endodontic sealers that are currently being used are totally nonirritating, yet without doubt they are being used with clinical success.
If over a short period of time (up to 30 days) a mild inflammation is present and it diminishes over time, a material with otherwise favorable properties can be considered biocompatible. Eluation of components was recognized by Ferracane and Condon and the inflammatory process as a result of this is the body’s response to irritation. Fibrous encapsulation without inflammation is the body’s response to isolate an otherwise biocompatible material. Furthermore, a material, usually small size particles, can be present in periapical tissues, cause no inflammation, and be present without encapsulation.
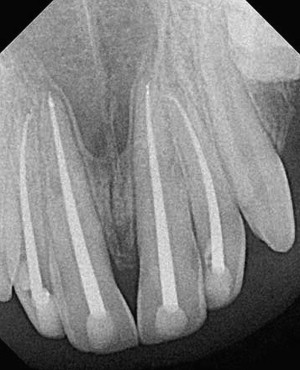
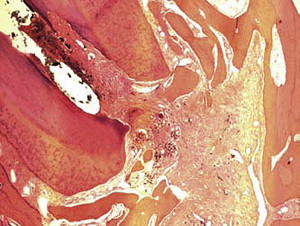
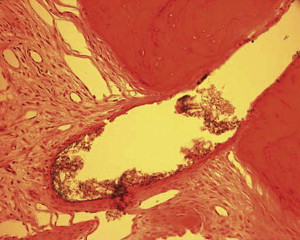
Fig. 1 is a representative radiograph of experimental sealers in 4 central incisors. After 113 days 2 reactions were observed for 2 different experimental sealers. Fig. 2 is an example of extrusion (intentional to determine biocompatibility) of the sealer into periapical tissues. The sealer particles are not encapsulated and no inflammatory reaction was observed. The periapical tissues reacted differently to the other sealer. After 113 days the histologic features of the apical area ( Fig. 3 ) showed slight extrusion into the periapical tissues. A fibrous encapsulation of the material can be observed, however, without the presence of inflammatory cells (magnification ×64, hematoxylin and eosin stain).
Leakage studies
Leakage of MBRS, whether coronal or apical, has been studied by numerous investigators, resulting in the publication of contradictory data that have generated more questions than answers.
It has been established that selection of an appropriate sealer will influence the outcome of endodontic therapy. For that reason many investigators have focused on this important aspect using techniques such as fluid filtration, dye penetration, and bacterial leakage tests. Frequently AH Plus or AH-26 are used as control materials. In one of the first published leakage tests using India ink, Zmener and Banegas reported no statistically significant difference between EndoREZ and AH Plus. Orucoglu and colleagues, using the fluid filtration method, reported that Diaket with cold lateral condensation leaked less apically than EndoREZ and AH Plus. However, others reported that AH Plus leaked less than EndoREZ and AH-26 using a single cone technique. Compared with zinc oxide-eugenol, MBRS was found to be more effective in sealing. These investigators also used the fluid filtration method. Using similar techniques, it was found that the apical seal of Epiphany and Resilon was not different from AH Plus and gutta-percha, AH Plus and Resilon, and Epiphany and gutta-percha. In contrast, using a fluid-transport method, Tunga and Bodrumlu concluded that Epiphany and Resilon leaked significantly less ( P <.05) than gutta-percha and AH-26. Others reached a similar conclusion when comparing Resilon and gutta-percha and AH Plus, and in bacterial leakage tests ; Epiphany and Resilon were superior to gutta-percha and various other sealers. Pitout and colleagues also used a bacterial leakage test and a dye penetration method and Biggs and colleagues did not observe a difference between Resilon and gutta-percha. Several investigators have used the dye penetration technique to demonstrate that MBRSs are superior or inferior to conventional materials. One explanation for the difference in results between the various MBRS materials can most likely be attributed to the presence or absence of moisture in the root canal at the time of obturation.
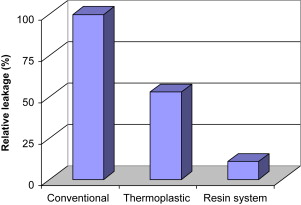
To put leakage studies in context, in 2001 Oliver and Abbott conducted a study to determine if there was a correlation between apical dye penetration and clinical performance of root fillings. They tested the length of apical dye penetration using a vacuum technique ex vivo in 116 human roots that had been root-filled at least 6 months before extraction. Endodontic treatment was classified as clinically successful or unsuccessful and the results for these groups were compared using an analysis of variance and the Student t -test. Positive and negative controls were used to test the experimental system. In unsuccessful cases the dye penetrated significantly further although the raw data suggested little difference. Overall, the dye penetrated in 99.5% of the specimens, and this indicates that the presence of dye in a canal is a poor indicator of whether a technique or material will succeed clinically. However, the extent of dye penetration may be related to the clinical outcome. The investigators concluded that clinically placed root canal fillings do not provide an apical seal that prevents fluid penetration and therefore the outcome of treatment cannot be predicted based on the results of apical dye leakage studies. In 1993 Wu and Wesselink reviewed the shortcomings of various tests reported in the literature. However, dye leakage studies may be useful to determine the performance of a new material or technique by conducting comparative studies with existing systems. An electrochemical technique that seems to be sensitive and has generated findings that correlate with bacterial leakage tests, has been published by von Fraunhofer and colleagues. Fig. 4 illustrates a comparison between resin sealers and conventional sealers.