Introduction
In this study, we evaluated the effects of surface modifications of miniscrew implants (MSIs) and force application on bone surrounding MSIs.
Methods
Seven skeletally mature male foxhound dogs were followed for 9 weeks; a randomized split-mouth design was used to compare 21 MSIs with sandblasted, large-grit, and acid-etched (SLA) surfaces and 21 identical machine-surfaced MSIs. MSIs immediately loaded with 200-g nickel-titanium coil springs were compared with unloaded MSIs. Bone volume to total volume ratios of cortical and noncortical bone regions were measured at 6 to 24 μm and 24 to 42 μm from the entire MSI surface using microcomputed tomography with an isotropic resolution of 6 μm.
Results
Clinical success of SLA-surfaced MSIs was 100%, compared with 85.7% for machine-surfaced MSIs. There was significantly ( P <0.05) more bone at the coronal aspects of the SLA-surfaced than the machine-surfaced MSIs; the SLA-surfaced MSIs also showed significantly greater decreases in bone between their most coronal and apical aspects. MSIs that were loaded demonstrated significantly ( P <0.05) greater decreases in surrounding bone than unloaded MSIs. The amount of bone within 6 to 24 μm of the MSIs was significantly less than that within 24 to 42 μm. Mean placement torque was higher for the SLA-surfaced (42 Ncm) than the machine-surfaced (39 Ncm) MSIs, but the difference was not statistically significant.
Conclusions
SLA surface treatment and loadings have significant effects on bone surrounding the MSIs; this might be related to higher success rates and greater secondary stability.
The small size, minimal surgical invasiveness, and relative affordability of miniscrew implants (MSIs) have added new dimensions to conventional orthodontic treatment methods. Despite their many advantages, the success rates of MSIs have yet to achieve those of endosseous implants. Some possible reasons attributed to the differences in failure rates include the use of implants with diameters less than 1.0 mm, peri-implant tissue inflammation, thin cortical bone, absence of keratinized mucosa, patient age, and the anatomic location of placement. One proven strategy to enhance success rates is to accelerate osseointegration and healing.
Titanium surface modifications and improvements to implant surface topography have been among the most important factors shown to enhance osseointegration of endosseous implants. The sandblasted, large-grit, and acid-etched (SLA) titanium alloy surface is a subtractive surface topography that has been well documented in the endosseous implant literature. Buser et al showed significantly greater shear strength of the bone-implant interface for the SLA-surface implants (1.14-1.54 Nm) than for the conventional machined titanium implants (0.15 and 0.25 Nm). The positive influence of SLA surfaces on the rate of osseointegration has also been demonstrated.
Whether forces applied to MSIs enhance osseointegration (ie, bone-to-implant contact) remains controversial. An effect might be expected because the structure of bone is dynamic, with its adaptation largely governed by biologic and mechanical interactions with the environment. The influence of force on the peri-implant tissue has been established for endosseous implant applications. However, research on the influence of a force load on osseointegration of MSIs with both biomechanical and histomorphometric analysis is limited and inconclusive.
The primary purpose of this study was to evaluate, by using microcomputed tomography (μCT), the effects of SLA surface treatment on the bone closely surrounding MSIs. The secondary aim was to evaluate the effects of loading on bone surrounding MSIs. μCT produces high-resolution 3-dimensional (3D) images for quantifying cortical and trabecular bone structures. It is nondestructive, and allows for the evaluation of uncontaminated and unprocessed specimens, evaluation of density and mineralization characteristics, and reconstruction of 2-dimensional and 3D views of bone.
Material and methods
Seven mature male foxhounds 1 to 2 years of age, weighing between 55 and 65 lbs, were used for this study. The Institutional Animal Care and Use Committee at Baylor College of Dentistry (Dallas, Tex) approved the care of the animals and the experimental protocols. Foxhounds are an established model for investigating peri-implant osseous dynamics; their larger mandibles provide sufficient space between MSIs for loading.
MSIs (IMTEC, Ardmore, Okla) with 2 material surface preparations were used. The MSIs were self-drilling and made of titanium alloy, 6 mm long and 1.8 mm in diameter. SLA-type surfaces and conventional machined surfaces were compared. These MSIs were used to reduce the possibility of contacting the lingual cortical plate during placement.
Each animal had 6 MSIs placed in the interradicular areas of the mandibular first and second molars. Two immediately loaded MSIs and 1 unloaded MSI were placed on each side. The SLA-surfaced MSIs were randomly allocated to 1 side; 3 machine surfaced MSIs were placed in the same positions on the opposite side.
Prior to MSI placement, alginate impressions were poured in die stone, and radiographic stents were fabricated from the models. On the day of placement, all animals were sedated with ketamine (2.2 mg/kg intramuscularly) and rompin (0.22 mg/kg intramuscularly) and given prophylaxis with ultrasonic scaling instrumentation. The stents were placed, and periapical radiographs were taken bilaterally to determine MSI placement locations. The animals were intubated and maintained with 1% isoflurane with oxygen at 1 L per minute. Gingival tissue around the surgical sites was irrigated and lavaged with 0.2% chlorhexidine irrigant before administration of local anesthesia (2% lidocaine with 1:100,000 epinephrine) via local infiltration. Radiographic stents were replaced and used as guides to ensure the accuracy of MSI placement. All 42 MSIs were placed in buccal alveolar bone perpendicular to the cortical plate or parallel to the occlusal plane ( Fig 1 , A ). The placement sites included the bifurcations of the first and second molars. Height and anteroposterior location of placement were measured on periapical radiographs. Unloaded MSIs were placed 4 mm apical to their loaded counterparts when no anatomic limitations were present. Placement sites were marked and prepared with a 1.5-mm tissue punch, and pilot holes were drilled by using a 1.1-mm surgical drill under copious irrigation. The MSIs were manually placed and tightened with a torque tester (Cedar Sugisaki Division, Northbrook, Ill). Placement torque was recorded for each MSI.
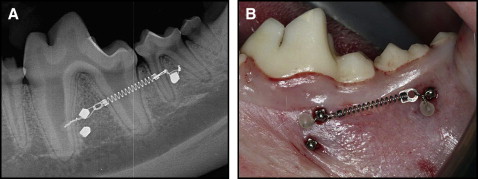
The MSIs were loaded with nickel-titanium 200-g coil springs tied through the islets on the heads of the implants with 0.010-in stainless steel ligature wire ( Fig 1 , B ). The activation forces of the coil springs were measured with a Correx Gram Force Gauge (Long Island Indicator Service, Hauppauge, NY). Postoperative peri-implant sites were irrigated with 0.2% chlorhexidine; periapical radiographs taken bilaterally were used to ensure proper MSI positioning. Both analgesics (torbugesic, 0.2 mg/kg [2 mg/mL with 1 mL per animal] and antibiotics (penicillin G, 60,000 units/kg) were administered. Inspections of appliance condition and prophylaxes with a tooth brush and 0.2% chlorhexidine irrigant were performed weekly.
Nine weeks after MSI placement, the animals were killed with 2 mL of Beuthanasia-D given intracardiac and perfused with 1 to 2 L of normal saline solution followed by 1 L of 70% ethanol. The mandibles were resected en bloc and stored in 70% ethanol before analysis.
Each bone-implant sample was retrieved with a 10-mm diameter trephine bur (ACE Dental Implant System, Brockton, Mass) under copious irrigation. The bur was modified to ensure that the bone-implant specimen fitted into the 9.8-mm inner diameter of the cylindrical specimen carriers used for scanning. The samples were trephined parallel to the long axis of the implant to ensure that all 3 layers of the bone (buccal cortical, medullary, lingual cortical) remained intact and undamaged during the retrieval process. The samples were stored in 70% ethanol.
A maximum of 3 bone-implant specimens were placed in cylindrical sample holders with 70% ethanol and evaluated by using μCT (μCT 35, Scanco Medical, Basserdorf, Switzerland) with an isotropic resolution of 6 μm. X-ray energy levels were set to 70 kVp, current to 114 μA, and integration time to 800 ms. A 0.5-mm aluminum filter and a high resolution setting of 1000 projections per 180° were used to ensure the highest quality scans with minimal metal implant artifacts.
A volume of interest (VOI) for each specimen was defined with the implant positioned in the center. The apical limit of the VOI was set to 10 slices (0.06 mm) coronally from the MSI tip; the coronal limit of the VOI was visually defined as the slice at which bone was observed to completely surround and contact the MSI. Because of the varying depths that each MSI was embedded in bone, the total number of slices in the VOI ranged from 936 to 1199, with an average total scanning time of 3.2 hours per specimen.
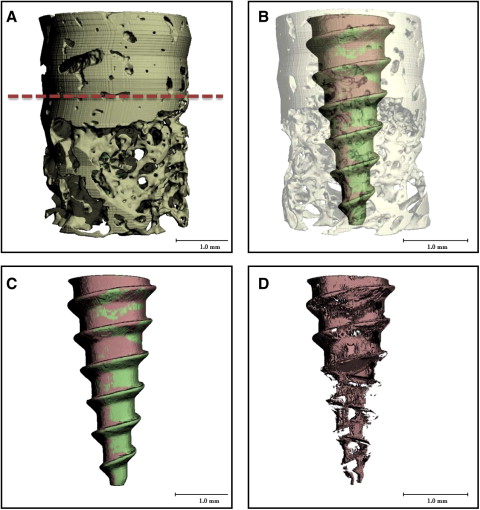
After scanning, a threshold (1 gray-scale “brightness” number, above which all voxels [ie, volumetric 3D pixels] are considered bone, and 1 gray-scale number below which all voxels are considered nonbone ) was determined based on multiple specimens to distinguish the MSI from mineralized bone and background. Based on comparisons with the original gray-scale images, threshold values that produced the best representation of the MSI, mineralized bone, and background images were selected. Three-dimensional images of the bone-implant specimens were reconstructed from the defined threshold parameters ( Fig 2 ). From the reconstructed 3D images, cortical and noncortical bone regions were identified; 2 Scanco Medical proprietary programming scripts with different parameters for bone-implant contacts were processed for each specimen.
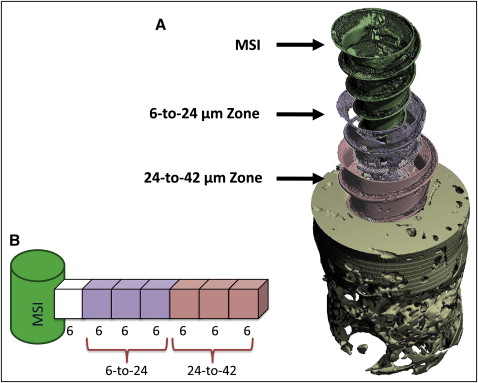
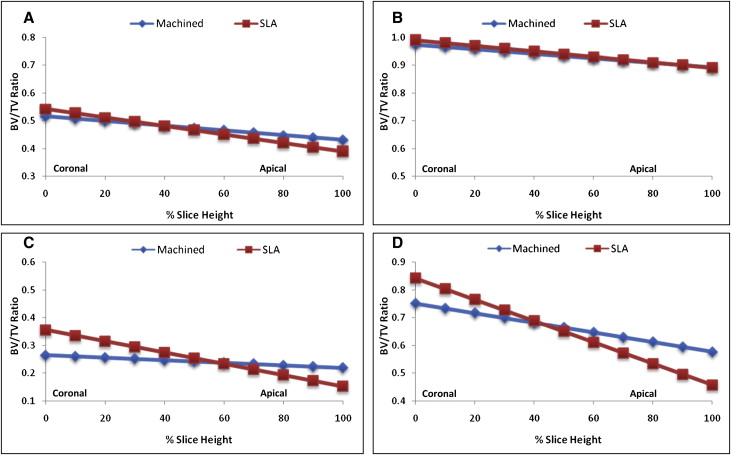
For each specimen, separate ratios of bone volume to total volume (BV/TV) were calculated for both cortical and noncortical bone regions. BV/TV measures the relative amount of bone per unit of volume; for example, a BV/TV ratio of 0.5 indicates 50% bone and 50% space, or nonbone. Although the cortical area included only cortical bone, the noncortical regions included mostly trabecular bone and limited amounts of cortical bone. The analyses pertained to 2 layers of bone, 6 to 24 μm and 24 to 42 μm, from the MSI surface ( Fig 3 ). Each layer was 18 μm thick and included three 6-μm voxels. The 24-to-42 μm layer was used because μCT segments beyond 24 μm from the MSI surface have been previously validated based on hisotologic sections of cortical (r = 0.65; P <0.05) and trabecular bone (r = 0.92; P <0.05). BV/TV ratios of bone 0 to 6 μm from the MSI were excluded in this study based on the assumption that the voxel immediately adjacent to the MSI was subject to metallic artifacts.
Statistical analysis
The analyses evaluated BV/TV surrounding the MSIs as a function of relative slice height. Each slice between the most coronal and apical aspects of the MSI was divided by the total number of slices to calculate relative slice height, which ranged from 0 (most coronal slice) to 100 (most apical slice). Relative slice height was calculated separately for the cortical and noncortical bone regions. Variations were partitioned between subjects and between slices within subjects. The linear relationships between BV/TV and relative slice height were estimated by using iterative generalized least squares. The analyses comparing the SLA and machine surfaces included only the loaded MSIs; the analyses comparing loaded and unloaded MSIs included both SLA-surfaced and machine-surfaced MSIs.
Results
The overall success rate for the study was 92.9%, with 3 of the 42 MSIs showing evidence of failure. There were 1 total failure and 2 partial clinical failures (slight mobility but stable enough for clinical use). The total failure pertained to an unloaded machine-surfaced MSI; the 2 partial failures were both loaded machine-surfaced MSIs, one with less than 1 mm of mobility, and the other with 1 mm of mobility. The partial failures were analyzed along with the remaining intact specimens. Clinically, the external surfaces of cortical bone for both partially failed implants had considerable amounts of remodeling, resulting in large bony concavities around the MSI. The loss of bone and consequent exposure of previously submerged threads might have compromised their stability.
The relative amounts of cortical bone in the 6-to-24 μm and 24-to-42 μm layers ranged from 39%-56% and 88%-100%, respectively. The amounts of noncortical bone in the 6-to-24 μm layers ranged between 15% and 36%; noncortical bone in the 24-to-42 μm layers ranged between 46% and 84%.
For both the cortical and noncortical regions, BV/TV decreased linearly and significantly ( P <0.05) between the coronal and apical aspects of bone ( Fig 4 ). The cortical and noncortical regions around the SLA-surfaced MSIs showed significantly greater amounts of bone at their most coronal aspects than did the machined MSIs ( Table I , Fig 5 ). Differences at the most coronal aspects ranged from 3.5% to 6.4% in the cortical regions and from 9% to 9.1% in the noncortical regions. The SLA-surfaced MSIs also showed significantly ( P <0.05) greater decreases in BV/TV than did the machined MSIs. Although the cortical regions showed few or no differences in the amount of bone in the apical aspects, the machine-surfaced MSI showed more bone than the SLA-surfaced MSIs in the noncortical regions.
| Machined | SLA | Difference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constant | SE | Linear | SE | Constant | SE | Linear | SE | Constant | SE | Linear | SE | |
| Cortical | ||||||||||||
| 6-24 μm | 4.98e-1 | 1.32e-2 | –9.28e-3 | 5.27e-5 | 5.62e-1 | 1.07e-2 | –1.54e-3 | 4.68e-5 | 6.44e-2 ∗ | 1.62e-2 | –6.16e-4 ∗ | 7.04e-5 |
| 24-42 μm | 9.65e-1 | 6.51e-3 | –6.30e-4 | 4.23e-5 | 1.00e+0 | 8.44e-3 | –1.17e-3 | 4.53e-5 | 3.51e-2 ∗ | 1.09e-2 | –5.40e-4 ∗ | 6.32e-5 |
| NonCortical | ||||||||||||
| 6-24 μm | 2.65e-1 | 2.29e-2 | –4.53e-4 | 3.95e-5 | 3.55e-1 | 2.27e-2 | –2.02e-3 | 4.04e-5 | 9.07e-2 ∗ | 4.43e-2 | –2.10e-3 ∗ | 7.97e-5 |
| 24-42 μm | 7.52e-1 | 3.14e-2 | –1.75e-3 | 5.45e-5 | 8.43e-1 | 3.12e-2 | –3.85e-3 | 5.80e-5 | 9.03e-2 ∗ | 3.22e-2 | –1.57e-3 ∗ | 5.67e-5 |
The cortical regions around the loaded MSIs showed greater amounts of bone than did the unloaded MSIs ( Fig 6 ). The differences at the most coronal aspect were statistically significant only for the 24-to-42 μm layer in the noncortical region ( Table II ; Fig 7 ). However, all loaded MSIs showed significantly greater decreases in BV/TV between the most coronal and apical aspects than did the unloaded MSIs, with differences ranging from a low of 1.8% (for the 6-24 μm layer in the cortical region) to 9.6% (for the 24-42 μm layer in the noncortical region).
| Unloaded | Loaded | Difference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constant | SE | Linear | SE | Constant | SE | Linear | SE | Constant | SE | Linear | SE | |
| Cortical | ||||||||||||
| 6-24 μm | 5.16e-1 | 1.24e-2 | –8.55e-4 | 5.63e-5 | 5.43e-1 | 1.14e-2 | –1.52e-3 | 4.46e-5 | 2.63e-2 | 1.78e-2 | –6.69e-4 ∗ | 7.21e-5 |
| 24-48 μm | 9.74e-1 | 8.87e-3 | –8.20e-4 | 5.28e-5 | 9.90e-1 | 6.87e-3 | –9.97e-4 | 3.93e-5 | 1.64e-2 | 1.13e-2 | –1.76e-4 ∗ | 6.50e-5 |
| Noncortical | ||||||||||||
| 6-24 μm | 2.92e-1 | 2.79e-2 | –9.18e-4 | 5.16e-5 | 3.14e-1 | 1.95e-2 | –1.28e-3 | 3.47e-5 | 2.20e-2 | 3.41e-2 | –3.60e-4 ∗ | 6.33e-5 |
| 24-42 μm | 7.28e-1 | 4.27e-2 | –2.04e-3 | 7.31e-5 | 8.24e-1 | 2.42e-2 | –3.00e-3 | 4.84e-5 | 9.55e-2 ∗ | 4.58e-2 | –9.56e-4 ∗ | 8.85e-5 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


