A wide range of human papillomavirus (HPV) genotypes have been detected in oral mucosa. Clinical infections with low-risk genotypes manifest as squamous papilloma, condyloma acuminatum, verruca vulgaris, or multifocal epithelial hyperplasia. Clinical infections with high-risk genotypes have been associated with malignant lesions. The most common genotype isolated from subclinical infection is HPV-16. A causal role for HPV in carcinogenesis of oral squamous carcinoma is minimal. Ongoing vaccination against HPV types 6, 11, 16, and 18 is expected to decrease the spread of infection and decrease the carcinogenic potential of HPV-16 in the oropharynx and oral cavity.
Key points
- •
A wide range of low-risk and high-risk human papillomavirus (HPV) genotypes have been detected in oral mucosa after infection. Clinical infections with low-risk genotypes manifest as squamous papilloma, condyloma acuminatum, verruca vulgaris, or multifocal epithelial hyperplasia.
- •
Clinical infections with high-risk genotypes have been associated with malignant lesions. The most common genotype isolated from subclinical infection is HPV-16.
- •
Unlike oropharyngeal carcinoma, a causal role for HPV in carcinogenesis of oral squamous carcinoma is minimal, with greater influence in a small subset of nonsmokers.
- •
Ongoing vaccination against HPV types 6, 11, 16, and 18 is expected to decrease spread of infection and decrease the carcinogenic potential of HPV-16 in the oropharynx and oral cavity.
Introduction
Human papillomavirus (HPV) is the cause of benign cutaneous and anogenital warts (condyloma acuminatum). HPV is also firmly established as an etiologic agent in cervical, vulvar, penile, and anal intraepithelial neoplasia (dysplasia) and carcinoma. Low-risk and high-risk anogenital HPV genotypes are spread by sexual contact. The same viral genotypes have been identified in oral condylomata, squamous papillomas, and head and neck squamous cell carcinomas. The same viral genotypes have also been detected in exfoliated cells of the oral cavity or brushings of normal oral mucosa in 6% to 30% of study populations; HPV-16, the high-risk genotype most commonly associated with cervical and genital carcinomas, is also the most common oral genotype identified. Although the incidence of oral squamous carcinoma in the United States, largely as a result of environmental carcinogens derived from tobacco, alcohol, and areca nut use has remained relatively stable, the incidence of oropharyngeal squamous carcinoma, strongly associated with HPV-16, has been increasing rapidly.
Introduction
Human papillomavirus (HPV) is the cause of benign cutaneous and anogenital warts (condyloma acuminatum). HPV is also firmly established as an etiologic agent in cervical, vulvar, penile, and anal intraepithelial neoplasia (dysplasia) and carcinoma. Low-risk and high-risk anogenital HPV genotypes are spread by sexual contact. The same viral genotypes have been identified in oral condylomata, squamous papillomas, and head and neck squamous cell carcinomas. The same viral genotypes have also been detected in exfoliated cells of the oral cavity or brushings of normal oral mucosa in 6% to 30% of study populations; HPV-16, the high-risk genotype most commonly associated with cervical and genital carcinomas, is also the most common oral genotype identified. Although the incidence of oral squamous carcinoma in the United States, largely as a result of environmental carcinogens derived from tobacco, alcohol, and areca nut use has remained relatively stable, the incidence of oropharyngeal squamous carcinoma, strongly associated with HPV-16, has been increasing rapidly.
The virus
HPVs constitute several species within 5 genera of the Papillomaviridae family. All HPVs share the same basic structure: a nonenveloped virus, 55 nm in diameter, with an icosahedral protein capsid consisting of 72 capsomeres. The viral genome is circular double-stranded DNA, approximately 7900 bp in length. All putative coding sequences are located on only 1 DNA strand.
The HPV genome consists of 3 functional regions. Soon after infection, early genes (E1-E7) are transcribed, and their protein products control viral replication and gene transcription and modulate epithelial cell growth and proliferation. The E2 gene product is a transcriptional repressor that inhibits transcription of oncogenic E6 and E7 proteins. Genes expressed later (L1 and L2) code for the capsid proteins and are transcribed before the final assembly of virions. An upstream regulatory region of the viral genome is noncoding and controls viral DNA replication and transcription of the early and late genes. Sequence analysis of the L1 major capsid protein gene derived from DNA isolated from various benign and malignant lesions has shown that considerable genomic variation exists in this gene. Less than 90% homology between L1 genes defines different HPV genotypes, and there are more than 120 well-characterized genotypes, with many more recognized ( Table 1 ).
| Genotypes | Lesion |
|---|---|
| 1, 2, 4, 26, 27, 57 | Verruca vulgaris |
| 3, 10, 26, 27, 75 | Verruca plana |
| 1, 2, 4, 63 | Plantar warts |
| 13, 32 | Multifocal epithelial hyperplasia |
| 6, 11, can also harbor high-risk genotypes | Condyloma acuminatum |
| 6, 11, can also harbor high-risk genotypes | Oral squamous papilloma |
| 6, 11, can also harbor high-risk genotypes | Recurrent respiratory papillomatosis |
| 6, 11, can also harbor high-risk genotypes | Anogenital low-grade intraepithelial neoplasia |
| 16, 18, 31, 33, 51, 52, 66 and other high-risk genotypes | Anogenital high-grade intraepithelial neoplasia |
| 16, 18, 31, 33, 35, 51, 66, 73, 82 and other high-risk genotypes | Anogenital and cervical carcinoma |
| 16, 33, 31, 18, 52 and other high-risk genotypes | Head and neck squamous cell carcinoma |
Much of the recent literature relating HPV genotypes with disease has focused on HPV in the cause of anogenital warts and dysplasia and cancer of the uterine cervix. At least 40 HPV genotypes can infect genital skin and mucosae. HPV genotypes associated with high-grade dysplasia and carcinoma of cervical, vaginal, vulvar, penile, and anal epithelium are subclassified as high risk, whereas those genotypes associated with low-grade dysplasia, condylomata, and other warts are described as low risk. High-risk types 16 and 18 account for approximately 70% of high-grade anogenital disease, whereas low-risk types 6 and 11 are the most common genotypes associated with condylomata and low-grade anogenital disease. In low-risk infections, the viral genome remains as an episome in the nucleus, independent of the host DNA. In this type of infection, E2 function is not disrupted, which allows E2 protein to potentially suppress transcription of E6/E7 gene(s). Replication of the viral genome occurs in parallel with host genome replication, resulting in a stable viral copy number distributed among daughter epithelial cells. In high-grade dysplasia and carcinomas, the high-risk HPV genome is present in high copy number, is typically integrated into the host DNA, and when integration involves disruption of the E2 gene and its regulatory function, there is increased transcription of E6 and E7 genes. Whether simply a quantitative increase or a significant structural difference in the E6 and E7 proteins produced by high-risk genotypes, or both, it is the increased production of these 2 oncoproteins in high-risk HPV infections that account for the carcinogenic potential of HPV.
E7 protein interacts with pRb, the tumor suppressor protein that controls entry into the S phase of the cell cycle. The E7:pRb complex effectively inhibits pRb control of these critical restriction points, allowing the rate of cell cycling to increase. Concomitantly, E6 protein interacts with several molecules in critical pathways that control cell replication, inhibiting mechanisms of suppression and enhancing the function of cellular oncoproteins. A major target of E6 is p53, the tumor suppressor protein that monitors critical cellular pathways and the integrity of the genome during DNA replication. DNA breakage, chromosomal loss, and mutations that potentially disrupt genome replication cause an increase in p53, which in turn halts the cell cycle to initiate DNA repair or, if irreparable, it initiates apoptosis. E6 protein inactivates p53 by targeting it for ubiquitination and degradation. With the resultant decrease in p53, defective host cell DNA can continue through the cell cycle. This process causes daughter cells to continually accumulate mutations and chromosomal loss caused by increased genetic instability, a hallmark of carcinogenesis. Inhibiting pRb function, as described earlier, accelerates the neoplastic transformation process.
HPV infection
HPV infection is limited to epithelium, with most infections occurring in squamous epithelium of skin and mucosae. After infection of basal epithelial cells and a variable incubation period, viral replication and assembly of virions occurs as squamous cells differentiate, with mature virus shed with sloughing superficial cells. Latent HPV infections are considered noninfectious, because the viral copy number per cell is too low to transmit disease. In subclinical HPV infections, viral DNA replication and transcription are active, present in infectious epithelium not yet observable as a clinical lesion. Clinical HPV infections contain active virus present in an infectious, clinically apparent lesion. Latent and subclinical infections are probably most common. The clinical and microscopic features of HPV-induced epithelial lesions vary with the anatomic site infected and the genotype of the virus. Thus, clinical lesions can be exophytic and flat, papillomatous or verruciform, or endophytic and less obvious. Histopathologically, subclinical and clinical lesions can be benign, dysplastic with varying degrees of intraepithelial neoplasia, or malignant. In clinical infections, virally affected epithelial cells can be visible microscopically in the upper spinous layer as koilocytes (cells with a small condensed nucleus and perinuclear clear space). Individual epithelial lesions can harbor more than 1 HPV genotype.
Persistent infection with high-risk HPV genotypes is necessary for HPV-induced carcinogenesis, but is not sufficient, because cervical cancer does not develop in most infected women. There are several factors that potentially govern the risk and rate of HPV-induced carcinogenesis. For example, a high-risk HPV infection that lacks effective translation of E6/E7 messenger RNA (mRNA) does not contribute significantly to carcinogenesis. Transcription of E6/E7 genes may be influenced by the degree to which E2 is disrupted after viral integration. Hypermethylation of genes in other important pathways governing host cell growth and replication may also decrease the effectiveness of E6 and E7 proteins, as might structural polymorphisms in p53 and pRb, which could decrease binding affinity of the viral oncoproteins. Furthermore, although high-risk infections that actively transcribe E6/E7 mRNA foster genetic instability, they themselves are not directly mutagenic. Other promoting agents, such as cigarette smoking, or coinfections, seem to be required.
HPV immunology
Because the growth cycle of HPV is limited to epithelium, HPV antigens are minimally exposed to the immune system. Nevertheless, cell-mediated immunity is believed to be the most effective means of controlling viral infection. All aspects of infection are increased in immunosuppressed individuals. Cell-mediated immunity is also believed to be the reason that most lesions and subclinical lesions undergo spontaneous regression. It has been reported that certain human leukocyte antigen (HLA) class II alleles that help govern the immune response are associated with persistence of viral infection and increased susceptibility for HPV-induced carcinogenesis.
Humoral immunity is limited, because the principal viral antigen, the L1 major capsid protein, is expressed only in the superficial epithelial layers. Seroconversion has been estimated to occur in only 60% of infected patients and can take 6 months or more to develop. Antibodies reactive to E6 and E7 proteins have also been detected and signify that the viral infection is transcriptionally active. Seropositivity for HPV16 E6 and E7 proteins has been used as a biomarker for increased survival of patients with oropharyngeal carcinoma.
HPV transmission
Transmission of HPV is caused by direct contact of skin or mucosa with an infected lesion. Minor trauma at the site is believed to facilitate infection of the basal epithelial cells. Cutaneous low-risk HPV infections include common warts (verruca vulgaris), juvenile flat warts (verruca plana), and plantar warts. They are widespread in the general population, with a prevalence of 3% to 20%. Verrucae are more common in children, whereas plantar warts are more common in adolescents and young adults. Anogenital warts (condyloma acuminatum) are transmitted via sexual intercourse, orogenital and manual-genital contact, and autoinoculation. Open mouth-to-mouth kissing is also a means of nonsexual transmission. Nosocomial transmission of HPV present in fumes caused by laser ablation of lesions is believed to be possible. HPV can be transmitted to the mouth and upper airway of a newborn from an infected mother during birth, which can result in recurrent respiratory papillomatosis and severe breathing difficulties in infants.
Epidemiology
It has been estimated that 6 million new genital HPV infections occur annually in sexually active young people. Genital HPV infection is one of the most common sexually transmitted diseases. Number of sexual partners, frequency of sex, and absence of condoms, immunization, and circumcision are important risk factors. The prevalence of anogenital warts in the general population is estimated at 1%, with 5% to 6% of a study group ranging in age from 18 to 59 years reported having had them.
The prevalence of oral HPV infection has also been studied using polymerase chain reaction (PCR) technique and subsequent genotyping of oral rinse samples or mucosal scrapings. The genotypes identified are typically the low-risk and high-risk types found genitally. Evaluation of rinse samples from a large study group in the United States ranging in age from 14 to 69 years showed that HPV DNA was detectable in 6.9% of the sample population. A separate study estimated that 7.3% of the adult US population had 1 or more HPV types detected in oral rinse. Low-risk, high-risk, and multiple type infections were found, and infection was more prevalent in men than women. High-risk infections were slightly more common than low-risk infections. High-risk HPV-16 infection was most common (1.0%). Oral infection was higher in those with a history of sexual contact and increased with number of sexual partners. Similar findings were reported in a smaller Finnish family study using scrapings from buccal mucosa analyzed by PCR and genotyping at various time points over 7 years. Infection of males (15%–31%) and females (15%–24%) varied during the 7-year period. Females showed a greater prevalence of low-risk infections than males, and like previous studies, the most frequent infection was HPV-16, and it was most likely to persist. Low-risk HPV infections cleared from the mouth faster than high-risk infections. Although the oral mucosa may play a significant role in HPV transmission, these methods provide no measure of viral load or whether the high-risk types present are transcriptionally active.
A recent endodontic publication reported HPV in a small percentage (13%) of periapical abscesses using PCR and a non–type-specific L1 sequence. Determination of the specific role of the virus in this location awaits further study.
HPV-16 has been detected in cyclosporine-induced gingival fibrous hyperplasia in renal allograft patients and in acute gingivitis in patients with AIDS. HPV-16 has also been detected in gingival tissues from patients with chronic periodontitis, and it has been postulated that oral, as well as sulcular and junctional epithelium, may serve as a source for infection or persistent infection of HPV. A recent study using real-time PCR with 104 gingival samples found no HPV-16 and concluded no association of HPV-16 with chronic periodontitis.
Subclinical HPV infections are not readily identified in the oral cavity by clinical means. Detection of high-risk genotypes in oral rinse samples by PCR and subsequent genotype-specific hybridization indicates only that HPV DNA (infection) is present. This information may provide impetus for patients to alter their social behavior, with subsequent tests evaluating persistence or absence of infection. This method does not provide information as to the location of oral infection, the surface area of oral epithelium infected, or the risk of transmission of infection to others by way of oral or sexual contact.
Oral clinical lesions associated with HPV
Verruca Vulgaris (Common Wart)
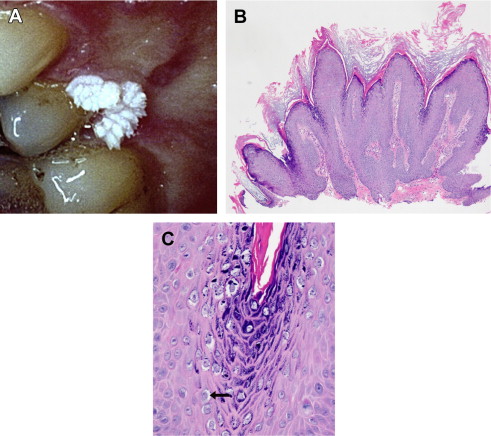
Caused principally by HPV-2 and HPV-4, this cutaneous lesion is most commonly observed in children on the hands and fingers. Verruca vulgaris is uncommon in the mouth ( Fig. 1 A). This wart is a well-circumscribed growth of squamous epithelium with prominent hyperkeratosis, giving it a white pebbly or papillary surface (see Fig. 1 B). A heavy granular layer and koilocytes are typically observed histologically (see Fig. 1 C). Oral lesions resemble skin lesions both clinically and microscopically and occur via autoinoculation of fingers to mouth.

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


