Fig. 2.1
(a) Histological section obtained from sound primary teeth. The tubular dentin (D) was synthesized and deposited by the odontoblasts (arrows), which are organized in a defined layer of cells that remains underlying this mineralized tissue. H/E, 32× (P Pulp). (b) High magnification of the (a). Note the continuous layer of odontoblasts beneath the thin layer of predentin. The subjacent pulp tissue (P) exhibits a number of cells, capillaries, and a loose extracellular matrix. H/E, 160× (D dentin)
Events that take place on the dentin reverberate to the pulp and vice versa [5].
The dentin–pulp complex is surrounded on the crown by dental enamel and on the root by cementum, periodontal ligament, and bone. The harmony of the complex is impaired if the surrounding tissues suffer some kind of injury that can reach the pulp by the root canal or through the dentinal tubules [5].
Although the tooth is a unique organ, the principles that guide its development are shared in common with other organs such as lung, kidney, heart, mammary glands, and hair follicles [6]. The most important developmental events are those guiding epithelial–mesenchymal interactions, which are characterized by a molecular cross talk between two tissues of different origins, the ectoderm and the mesenchyme [6].
Different stages of tooth development have been recognized at a microscopic level by their histologic appearance and were classically described as the dental lamina, bud, cap, and the early and late bell stages. In the modern literature, a functional terminology has been used to describe odontogenesis into four phases: initiation, morphogenesis, cell differentiation (cytodifferentiation), and matrix apposition [6]. The dental lamina is the first sign of tooth development. At the lamina stage, cells of the dental epithelium and those of the underlying ectomesenchyme divide at different rates and continue to grow and thicken to form a bud [6]. At the bud stage, cells of the ectomesenchyme proliferate and condense to give rise to the dental papilla. These cells have increased ability to proliferate, mobilize, and differentiate.
The morphogenetic phase involves the stages of bud, cap, and initial bell phase. During this period, a number of ectomesenchymal cells adjacent to the epithelium increase inside the ectomesenchyme, producing the site of origin of the dental papilla and of the dental follicle. These will develop into the dentin–pulp complex and into the support tissues of the tooth, respectively [6]. The formation of the enamel knot, during the transition from bud to cap, marks the beginning of crown formation. The cells of the enamel knot do not grow and serve as a signal for the cuspid formation pattern, influencing the form of the crown and the development of the dental papilla [6].
During the initial bell phase, the epithelium cells assume different morphologies, giving rise to the enamel organ, also called dental organ. This enamel organ is composed of the following four different stratums: internal enamel epithelium, stratum intermedium, stellate reticulum, and external enamel epithelium.
The internal epithelium of the enamel organ interacts with the undifferentiated superficial mesenchymal cells (also known as embryonic cells) of the dental papilla to form enamel, dentin, and pulp. Overall, cells of the internal dental epithelium elongate and become highly columnar, starting the late bell stage (phase of cytodifferentiation). This modification of the internal dental epithelium cells serves as a signal for the peripheral mesenchymal cells of the dental papilla, which after lining the basement membrane differentiate and assume an elongated morphology with odontoblast phenotype [6]. This phenomenon, characterized as differentiation of odontoblasts, has been intensely studied, leading to important advances in the knowledge of pulpal biology, particularly related with the mechanisms of healing of this specialized connective tissue against injuries and different pathological stimuli. More details about this issue will be discussed later in this chapter.
It is important to know the basic process of differentiation of the superficial undifferentiated mesenchymal cells from the dental papilla by stimuli expressed by cells of the internal dental epithelium. Growth factors, particularly those belonging to the superfamily TGF-β, are expressed by these epithelial cells. While the mesenchymal cells of the most superficial region of the dental papilla become competent, the last mitosis of the mother cell is positioned adjacent to the basal membrane. The daughter cells remain in the internal area of the papilla and will be part of the cell-rich zone that is clearly observed in the mature pulp. At this point both mother and daughter cells are referred as pre-odontoblasts because they assume the competency to differentiate into odontoblasts. Between the internal dental epithelium cells and the pre-odontoblasts is the basal membrane, which is composed of collagen, laminin, heparin sulfate, and other proteoglycans.
This basal membrane has an important role in the reciprocal activation of the epithelium/mesenchyme, resulting in a variety of epigenetic interactions, determining the phenotype of the odontoblasts. After the epithelial cells secrete the growth factors of the TGF-β superfamily, these bioactive proteins remain attached to the basal membrane. The components of the basal membrane activate these TGFs to interact with membrane receptors of the pre-odontoblasts. The translation of these signals results in the activation of the pre-odontoblasts, which start to secrete more growth factors and express the msxs genes. The final differentiation of the pre-odontoblasts into odontoblasts occurs only after the interaction of the fibronectin, which is deposited on the basal membrane and the 165 KDa pre-odontoblasts membrane receptors. The sequence of differentiation of the dental papilla mesenchymal cells into odontoblasts until the beginning of the synthesis and deposition of collagen-rich dentinal matrix is presented in Fig. 2.2.
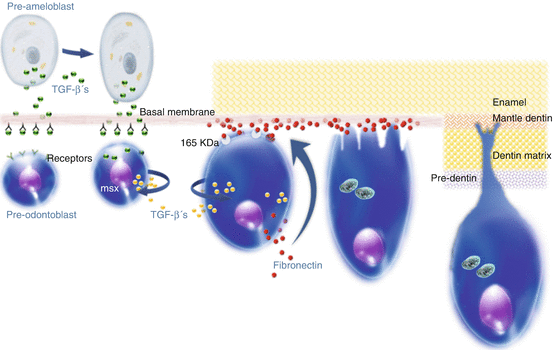

Fig. 2.2
Mechanism by which the pulpal mesenchymal cells present in the periphery of the dental papilla are differentiated into odontoblasts (Adapted from [31]) (J. Hebling, 2015)
The odontoblasts synthesize the dentinal organic matrix both adjacent to the cellular body and close to the mineralization fronts. During the period of upregulation of the TGFs, the pre-odontoblasts start to synthesize fibronectin and express the membrane protein 165 KDa, which is required to interact with the fibronectin. The msxs homeoproteins are probably involved in the reorganization of the pre-odontoblast cytoskeleton, which plays an important role in the process of differentiating into elongated cells, referred to as odontoblasts.
The mineralization process is mainly dependent on the odontoblasts’ activities: they release phospholipids and alkaline phosphatase containing vesicles that produce the hydroxyapatite crystals. The dentin matrix mineralization is heterogeneous, by globular calcification, resulting in fronts of mineralization or calcospherites. With continuous growth, the crystals tend to fuse (secondary mineralization), forming a mineralized mass around the odontoblastic processes, granting the dentin a tubular aspect (system of dentinal tubules). The portion of the dental papilla that is involved with dentin becomes the dental pulp. The odontoblasts form the dentin, but depend on it to become the pulp. The direction of the odontoblasts is towards the center, in relation to the dental pulp, and the decrease of the internal space favors a curved path of these cells. Once the first layer of dentin is formed, the cells of the internal epithelium (pre-ameloblasts) elongate and differentiate into ameloblasts starting to produce the enamel organic matrix, which becomes mineralized almost instantaneously.
As dentin is formed, the most cervical cells of the internal epithelium of the enamel organ become pre-ameloblasts, an event that occurs from the incisal (or from the cuspids) to the cervical area. When the dentin formation approximates the cervical loop, the cells of internal and external epithelium of the enamel organ proliferate from the loop, forming a double layer of cells, also known as Hertwig’s epithelial root sheath. The expansion of the sheath is followed by the formation of the radicular dentin. The cells of the dental follicle closer to the external layer of the sheath differentiate into cementoblasts and start to produce the cementum organic matrix; the follicle also produces the periodontal ligament and alveolar bone. There is a free border on the epithelial sheath, the epithelial diaphragm, which closes slowly as the root is formed (Fig. 2.3). As long as the apex of the root is not totally formed, there will be dental papilla, composed of ectomesenchymal cells. This has clinical relevance, because part of these cells can remain vital even after pulp necrosis. In this specific condition, it is possible to have a continuous apexogenesis or the formation of the radicular apex with dentin. When there is a severe reduction in the number of ectomesenchymal cells in the dental papillae, the tooth can still have apexification induced by an intracanal dressing [5]. This issue will be dealt in detail in Chap. 8.
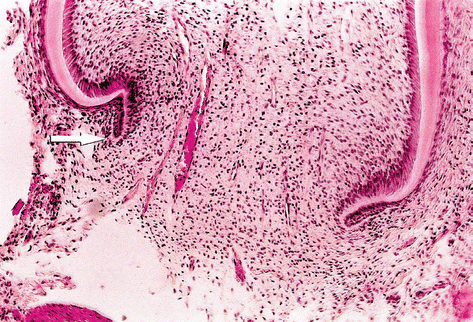

Fig. 2.3
Histological aspect of the formation of the apex. Hertwig’s sheath, involved by ectomesenchymal cells of the papilla, can be seen on the left (Courtesy Prof. Roberto Holland, 2008)
2.3 The Dental Pulp
The dental pulp is a specialized connective tissue confined between rigid walls of mineralized tissues (dentin, enamel, and cementum). The dental pulp can communicate with the external environment of the tooth through the apical foramen, foramina, and/or lateral canals providing the pulp with a low-tolerance environment, because the nutritional substrate comes from the vascularization that passes through the small foramens and foramina.
Loose connective tissue forms the stroma (nutritional supportive tissue) and the parenchyma (functional tissue) of several organs of the human body. In the pulp, this loose connective tissue forms both the stroma and the parenchyma at the same time, as it sustains itself and the dentin substrate and produces dentin. With the production of dentin, the pulp remains enclosed within the central part of the tooth, having a coronal and a radicular portion. In uni-radicular teeth, the coronal and radicular pulp tissues are contiguous, but in multi-radicular teeth, the floor of the pulp chamber has a clear distinction: the coronal pulp is rich in cells and extracellular matrix, while the radicular pulp has more fibers, and the vascular–nervous sheath is more concentrated, with less anastomosis (Fig. 2.4).
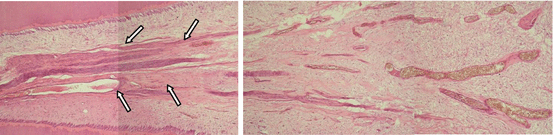

Fig. 2.4
Sections obtained from human sound teeth showing the radicular (left) and coronal (right) portions of the pulp tissue. Note the fibrous connective pulp tissue with vascular–nervous sheath close to the apical foramen (arrows). Conversely, the coronal pulp exhibits loose connective tissue with a number of blood vessels. H/E, 32×
2.3.1 Odontoblasts
The odontoblasts have been traditionally described as cells lining the periphery of the pulpal space and extending their cytoplasmic processes into the dentinal tubules. These cells have several junctions, which allow for intercellular communication and help to maintain the relative position of one cell to another. In young permanent teeth, the pulp tissue exhibits defined zones. The cell-free zone is located just below the odontoblastic layer and contains an extensive plexus of unmyelinated nerves and blood capillaries. The cell-rich zone, which presents a number of undifferentiated mesenchymal cells, is observed adjacent to the cell-free zone. The core of the dental pulp contains larger blood vessels and nerves, which are surrounded by large area of extracellular matrix. This pulp morphology is similar to that observed in primary teeth, but the zones are not so well defined (Fig. 2.5a, b).
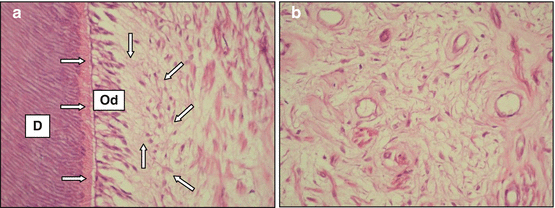

Fig. 2.5
(a) Sections obtained from human sound primary teeth. Note the presence of tubular dentin (D), predentin (horizontal arrows), odontoblast layer (Od), cell-free zone (vertical arrows), cell-rich zone (oblique arrows), and the core of the pulp. H/E, 160×. (b) The core of the pulp tissue contains a number of blood vessels and nerves, which are surrounded by a large area of extracellular matrix. H/E, 160×
Although this description is correct during active dentinogenesis, it is now accepted that the size of the odontoblasts and the content of their cytoplasmic organelles vary throughout their life cycle and are closely related to their functional activity. The relationship between the size of the odontoblasts and their secretory activity can be demonstrated by differences in their size in the crown and in the root, and different dentinogenic rates may be expressed in these two areas of the tooth [7].
The odontoblasts are highly specialized cells and are responsible for the formation of dentin. Due to the extension of their cytoplasmic processes into the dentinal tubules, these cells compose the main part of the dentin–pulp complex. When this complex is damaged by disease or attrition, or is affected by operative procedures, it reacts in an attempt to defend the pulp tissue.
2.4 Dentin Structure and Composition
Dentin is the most abundant mineral component of the teeth. It is composed of 70 % inorganic crystals (hydroxyapatite), 20 % collagen fibers and other proteins, and 10 % water (all by %volume). Dentin can be classified as primary, secondary, or tertiary, according to the time of development and the histological characteristics of the tissue.
2.4.1 Types of Dentin
The primary dentin is composed by the mantle dentin and by the circumpulpal dentin, which are physiological structures deposited up to the eruption of the tooth and its contact with the antagonist. Mantle dentin is the first dentin to be formed. It is deposited along the enamel–dentin or dentin–cementum junction, parallel to the tissues coating it. It is almost totally free of developmental defects. The odontoblasts, when supported by the basal membrane, have several cytoplasmic projections. This results in mantle dentin that is highly branched on the periphery converging into a single prolongation towards the center of the pulp chamber. Mantle dentin has an approximate thickness of 80–100 μm. The circumpulpal dentin is formed after the deposition of the mantle dentin and constitutes the majority of the dentin. Since the odontoblasts produce the organic dentin matrix centripetally concerning the pulp, the space available becomes increasingly reduced leading to an “S”-shaped curvature in the circumpulpal dentin, which is more pronounced at the crown and more discrete at the root [5].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


