Fig. 9.1
Human dental pulps engineered with human dental pulp stem cells (DPSCs). Postnatal stem cells (DPSCs) were seeded in tooth slice/scaffolds and transplanted into the subcutaneous space of immunodeficient mice. Photomicrographs of engineered pulps 2 or 3 weeks after transplantation. To identify the human DPSCs, we stably transduced them with the LacZ gene that makes these cells blue in response to staining with β-galactosidase
Pulp-related stem cells can also be harvested from dental papilla, the organ that generates the dental pulp during tooth development. These stem cells from apical papilla, which give rise to the odontoblasts that are responsible for root dentin formation, can be harvested from developing wisdom teeth, and they show a high capacity for dentin regeneration, as they are likely to be less differentiated compared with DPSCs [20, 21].
Tooth-derived mesenchymal stem cells express STRO-1, a putative mesenchymal stem cell marker; their potential to differentiate into odontoblasts, osteoblasts, adipocytes, and neural cells has been demonstrated [8–10, 13, 15, 22]. DPSCs were also shown to be positive for alpha-smooth muscle actin, CD146, and pericyte-associated antigen (3G5) [12]. Notably, it was recently demonstrated that dental pulp stem cells reside in perivascular niches [23].
9.4 Growth and Differentiation Factors
Enhanced understanding of the biological processes involved in both tooth development and repair are creating conditions to enhance reparative responses and tissue regeneration. The dentin matrix contains innumerous proteins and growth factors capable of stimulating tissue repair. These growth factors are secreted by pulp cells and deposited within the dentin matrix during mineralization [24, 25] where they remain protected in active forms. Demineralization of the dentin after application of cavity-etching agents or dressing for pulp capping [26], or even during cavity preparation [27, 28], releases these growth factors and makes them available to the surrounding cells. They seem to play key roles in controlling many of the events of reparative dentin formation [10, 29]. Growth factors, especially those of the transforming growth factor (TGF)-beta family, are important players in cellular signaling; they stimulate odontoblast differentiation and dentin matrix secretion, leading to increased reparative dentin formation [29].
Another important family of growth factors in tooth development and regeneration are the bone morphogenetic proteins (BMP). Recombinant human BMP-2 stimulates the differentiation of adult pulp stem cells into an odontoblast-like cell type in culture [30, 31]. Similar inductive effects of TGF-beta 1–3 and BMP-7 have been demonstrated in cultured tooth slices [32, 33]. Recombinant BMP-2, BMP-4, and BMP-7 induce formation of reparative dentin in vivo [30, 34]. The application of recombinant human insulin growth factor (IGF)-1 has been found to induce dentin bridging, comparable to a calcium hydroxide-based capping agent [35]. In addition to growth factors, other molecules have been shown to stimulate pulp cell differentiation. Dentin matrix protein (DMP)-1, a noncollagenous protein involved in mineralization processes, induces cytodifferentiation, collagen synthesis, and calcified deposits after direct pulp capping in animal models [36]. Dexamethasone, a synthetic glucocorticoid, reduces cell proliferation and induces expression of alkaline phosphatase and dentin sialophosphoprotein in primary human dental pulp cells [37]. The addition of beta-glycerophosphate to the cell culture medium in explants from human teeth induced a change in cell morphology, collagen synthesis, and mineral formation [38]. Combinations of dexamethasone with inorganic phosphate have been used for dental stem cell differentiation and the induction of mineralization in several studies [8, 9]. The key message here is that there are known biological regulators of dental pulp cell behavior that have been considered as potentially viable therapeutic strategies for regenerative endodontic procedures.
9.5 Scaffolds
Scaffolds are carriers for cells, which provide a 3D environment for cell adhesion, migration, and differentiation. Ideally, they should biodegrade at the same rate as the new tissues form. Scaffold materials should be biocompatible and nontoxic. For dental pulp tissue engineering, the injectable materials are advantageous. Natural polymers, such as collagen, elastin, glycosaminoglycans, fibrin, alginate, silk, and chitosan, have long been used as carriers [39]. Although these materials provide structural strength and are biocompatible and biodegradable, they offer few possibilities for controlling the structure or making compositional modifications to improve their performance. Synthetic polymers, on the other hand, provide high control of the mechanical and chemical properties. Polylactic acid (PLA), polyglycolic acid (PGA), and their copolymer poly(lactic-co-glycolic acid) (PLGA) have been widely used for tissue engineering applications including regenerative dentistry [40, 41]. Hydrogel is particularly interesting because of their tissue-like viscoelastic properties, efficient transport of nutrients and metabolic products, uniform cell encapsulation, and the possibility of injection and gelation in situ. Gels have been made from polyethylene glycol fibrin, glycosaminoglycans, or peptide-based building blocks. Based on their chemistry, they can be chemically or physically cross-linked, and modifications such as the incorporation of biofunctional molecules or growth and differentiation factors are possible [42].
9.6 Tissue Engineering in Endodontics
The concept of tissue engineering was conceived by Langer and Vacanti in the early 1990s to describe the technique for biological tissue regeneration [43]. Tissue engineering refers to the science of generating new living tissues to replace, repair, or augment the diseased/damaged tissue and restore tissue/organ function [44].
The objectives of tissue engineering procedures in endodontics are to regenerate a pulp-like tissue and ideally the pulp–dentin complex. An immature tooth with a necrotic pulp does not have residual progenitor pulpal cells to continue root development [45–47]. The main goals of conventional root canal therapy, including complete cleaning and shaping and appropriate obturation, cannot be achieved in an immature root. Moreover, the short, weak, and fracture-prone root of an immature tooth [48] becomes even weaker after mechanical instrumentation of the root canal. Apexification with Ca(OH)2 [49] or an apical plug [50] may solve many of the problems associated with conventional root canal therapy; however, there is still the risk of horizontal root fracture especially at the cervical area and tooth mobility due to compromised crown-to-root ratio (Chap. 8).
Apexification with Ca(OH)2 was proposed by Frank (1966), in which Ca(OH)2 is used in multiple visits during a long period of treatment to induce an apical barrier [49]. In addition to the extended treatment period, long-term contact of dentin with Ca(OH)2 decreases the mechanical strength of dentin, making the tooth susceptible to fracture [51, 52]. Cvek (1992) reported a higher incidence of cervical root fracture in root-filled immature teeth compared to mature teeth following dental trauma [48]. Therefore, preserving (or restoring) the pulp vitality of immature teeth with deep caries or dental trauma is of great importance.
To keep the tooth vital, several research groups are pursuing the idea of engineering dental pulp, which was initially proposed by David Mooney’s group at the University of Michigan in the nineties [53, 54]. Dental pulp fibroblasts were cultured on PGA scaffolds for 60 days, and the cells were proliferated and formed tissue similar to native dental pulp [53]. Bohl et al. tested whether different scaffold materials influenced the proliferation and collagen synthesis of dental pulp fibroblasts 2 years later and found PGA to be the most conducive compared with alginate and collagen type I hydrogel [54]. A dental pulp-like tissue was generated using DPSCs in combination with collagen scaffolds and DMP-1 as a morphogenic factor on dentin slices after transplantation in vivo [55]. Our group used SHED cells seeded onto polymer scaffolds within tooth slices and transplanted these constructs into immunocompromised mice. A pulp-like tissue formed within the tooth slices after 2–4 weeks, the cells lining the dentin showed expression of odontoblast/osteoblast markers, and microvessel formation was observed [16]. Notably, SHED differentiated into odontoblasts that synthesized new tubular dentin, confirming their differentiation into fully functional odontoblastic cells [17].
Galler’s group demonstrated the growth and differentiation of stem cells derived from different dental tissues in a PEGylated fibrin hydrogel. The cells degraded the fibrin, replaced it with a collagenous matrix, and deposited mineral after osteogenic induction. Furthermore, SHED and DPSCs were tested for compatibility with a self-assembling peptide hydrogel, which offers several possibilities to modify the matrix. The optimization of a peptide sequence through the incorporation of the cell adhesion motif RGD (arginine–glycine–aspartic acid) and a matrix metalloproteinase (MMP)-2-specific enzyme cleavable site led to increased proliferation rates and enhanced migration of SHED and DPSCs into these matrices [56]. Furthermore, studies with peptide-based hydrogels showed that the differentiation of dental pulp stem cells can be stimulated in this type of matrix [57]. Further steps toward a tailor-made system include the incorporation and slow release of growth and differentiation factors, such as TGF-beta1, FGF2, and VEGF.
9.7 Approaches for Regenerative Endodontics
The following are possible approaches (Fig. 9.2) that have been explored for dental pulp tissue regeneration: (A) root canal revascularization via blood clotting, (B) postnatal stem cell transplantation using injectable scaffolds, and (C) pulp transplantation using stem cells grown in sheets. These techniques are based on the basic tissue engineering principles and include specific consideration of stem cells, growth factors, and scaffolds. Of these approaches, the only one that is currently approved for general clinical use in the USA and in several other countries throughout the world is the “root canal revascularization via blood clotting” procedure, as called simply “revascularization” (see Chap. 8). Nevertheless, the approaches employing stem cell transplantation are areas of intense basic and translational research that should lead to clinical transplantation in the not so distant future.
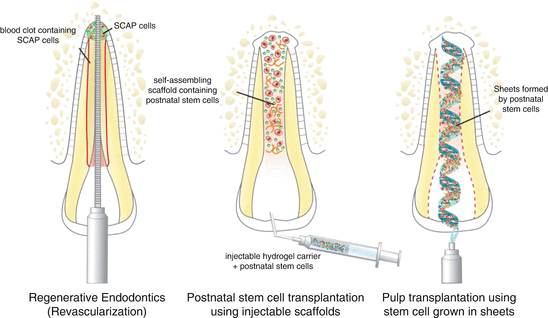

Fig. 9.2
Current strategies for the engineering of dental pulps. Diagram depicting three strategies for the engineering of human dental pulps, as follows: (a) root canal revascularization via blood clotting, (b) postnatal stem cell transplantation using injectable scaffolds, and (c) pulp transplantation using stem cells grown in sheets
9.7.1 Root Canal “Revascularization” via Blood Clotting
Nygaard Ötsby (1961) first described the concept of revascularization, indicating sustained root development following blood clot induction in the root canal of immature teeth suffering from pulp necrosis. Accordingly, the ideal outcome for teeth with an immature root and necrotic pulp would be formation of vascularized tissue in the canal space capable of inducing normal root development [58, 59].
The regeneration process depends on the presence of osteoblast and odontoblast progenitor stem cells in the apical dental papilla, which might be resistant to infection and necrosis within the canal due to their vicinity to periodontal blood vessels [60]. The aim is to create a suitable environment so that the periapical stem cells can proliferate into the root canal space for regeneration of pulp tissue and continuation of root development [61].
This technique involves the instrumentation of the root canal beyond the apex with the objective of forming a blood clot. It involves the use of intracanal irrigants (e.g., diluted sodium hypochlorite, chlorhexidine) with placement of antibiotics (a mixture of ciprofloxacin, metronidazole, and minocycline paste) for several weeks. This particular combination of antibiotics effectively disinfects the root canal system and increases revascularization of the necrotic tooth [62, 63]. This approach is being increasingly used as it is relatively simple and can be completed using standard instruments and medicaments. Notably, the regeneration of tissue in root canal systems occurs by a patient’s own blood cells avoiding the possibility of immune rejection or pathogen transmission from replacing the pulp with a tissue-engineered construct.
Despite the simple technique of revascularization, several concerns still need to be addressed. Most of the support for this technique comes from case reports or studies with small number of cases. This treatment typically leads to rather unpredictable responses. More definitive studies with longer follow-up times are necessary to determine more convincingly the true success rate of this procedure and define better the indications for this treatment approach. Nevertheless, this is an approved clinical procedure for regenerative endodontics that offers the possibility of treating in a biological way young teeth with necrotic pulps.
9.7.2 Postnatal Stem Cell Therapy Using Injectable Scaffolds
A possible method for treatment of necrotic young permanent teeth with open apex involves the transplantation of postnatal stem cells (e.g., SHED, DPSC) into the disinfected root canal system [3]. Postnatal stem cell can be derived from multiple tissues, including the skin, buccal mucosa, fat, bone, and dental pulp [64]. These cells will have to be transplanted into the root canal with the use of a scaffold material.
Rigid tissue-engineered scaffold provides excellent support for cells used in bone and other body areas where engineered tissue is required to provide physical support [65]. However, in root canal system, a tissue-engineered pulp is not required to provide structural support of the tooth. This will allow tissue-engineered pulp tissue to be administered in a soft 3D scaffold matrix such as hydrogel. Hydrogel is an injectable scaffold that can be delivered by syringe [66]. The hydrogel has the potential to be noninvasive and easy to deliver into the root canal system. It promotes pulp regeneration by providing a substrate for cell proliferation and differentiation into an organized tissue structure [67]. To make hydrogel more practical, research is focusing on making them photopolymerizable to form rigid structures once they are implanted into the tissue site. Work from the Galler laboratory [68] is focused on the development of functionalized injectable scaffolds that can be used for regenerative endodontic approaches. Indeed, this is a very exciting area of research and a tremendous opportunity for collaborations between clinicians, material scientists, and cell biologists.
We have shown that the implantation of SHED cells into full roots of premolars transplanted in the subcutaneous of immunodeficient mice results in the regeneration of functional dental pulps throughout the extent of the root canal [69]. This was particularly successful when we used PuraMatrix as an injectable scaffold for cell delivery. In contrast, transplantation of SHED cells in recombinant human collagen type I matrices was not as successful, as demonstrated by the presence of areas of odontoclastic activity. Ongoing clinical trials conducted by Dr. Misako Nakashima and colleagues in Japan are beginning to explore the safety and efficacy of postnatal stem cell transplantation for the treatment of necrotic permanent teeth.
9.7.3 Pulp Transplantation Using Stem Cells Grown in Sheets
In this case, replacement pulp tissue is generated in vitro and then transplanted into a clean root canal of a necrotic tooth. The cellular source for the replacement pulp tissue could be cells taken from a biopsy or an exfoliated primary tooth that has been expanded in the laboratory. The cultured pulp tissue is grown in sheets in vitro on biodegradable polymer nanofibers or in a sheet of extracellular matrix protein such as collagen I or fibronectin [70]. These sheets of pulp-like tissue would then be rolled to form a cylinder that can be implanted into the disinfected root canal. These sheets are not very difficult to grow and are likely more stable than an injection of dissociated cells. However, sheets of cells lack vascularity, and therefore the apical portion of the canal has the highest likelihood to be conducive to the survival of the cells in these constructs [71]. Another potential challenge is the fact that these cellular sheets are extremely fragile, making it difficult to place them in the root canal without breakage. Hence, this approach still needs to be further optimized prior to consideration for clinical use. Notably, the Sfeir group recently demonstrated that dental pulp stem cells in 3D self-assembled scaffoldless sheets can be used to regenerate a vital pulp-like tissue in tooth root canal systems. These recent data suggest a promising new method to deliver stem cells into root canals for pulp regeneration therapies [72].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


