Abbreviation
Chemical formula
Ca/P
Solubility K sp at 25 °C
MCPM
Ca(H2PO4)2.H2O
0.5
18 g/L
MCPA
Ca(H2PO4)2
0.5
DCPD
CaHPO4.2H2O
1.00
0.32 g/L
2.39 × 10−7
DCPA
CaHPO4
1.00
OCP
Ca8(HPO4)2(PO4)4.5H2O
1.33
1.05 × 10−47
TCPa
Ca9[]1(HPO4)(PO4)5(OH)[]1
1.50
CDA
Ca9.5[]0.5(HPO4)0.5(PO4)5.5(OH)0.5[]1.5
1.58
β-TCP
Ca3(PO4)2
1.50
2.83 × 10−30
HA
Ca10(PO4)6(OH)2
1.67
3.37 × 10−58
Human bonea
Ca8.3[]1.7(PO4)4.3(HPO4,CO3)1.7 (OH,1/2CO3)0.3[]1.7
1.55 – 1.75
7.2 × 10−53 − 6.4 × 10−58
The sintering of commercial CaP reagents (labeled as “hydroxyapatite” or “calcium phosphate, tribasic” or “tricalcium phosphate”) above 900 °C was shown to result in pure HA, pure β-TCP, or BCP (Table 4.1).
Recent developments related to CaP scaffolds including improvements in terms of engineering chemistry, surface properties, microstructure, and porosities, which lead them to be considered as being bioinstructive rather than osteoconductive scaffolds, have opened up new opportunities for bone regenerative technologies [8]. Not only are some of these CaP bioceramics scaffolds osteoinductive in their own right, but evidence also supports the hypothesis that specific engineering bioceramics have a direct influence on the differentiation and proliferation of human mesenchymal stem cells (hMSCs). Tissue engineering, new bioactive molecules, and new surgical technologies increase the potential application of CaP bioceramics as carriers of these cells and also as scaffolds capable of guiding the behavior of these cells and the efficiency of bone regeneration [8].
4.2 Definitions
4.2.1 Calcium Phosphate Bioceramics
For over 30 years, HA and related CaPs have been widely used in the field of biomaterials as bone graft substitutes [3, 6, 7, 9, 10]. These materials have clinical applications in orthopedic, spinal, and maxillofacial surgery. They are currently used in various forms, but macro-/microporous bioceramics are the most advanced products [11]. Bioceramics are manufactured from well-characterized CaP powders that are mixed with pore makers and sintered at elevated temperatures (e.g., 1,000–1,300 °C). Research has primarily focused on both the formulation of appropriate bioceramic chemistry and the optimization of the physical pore structure. Mastering the chemistry of CaP bioceramics is clearly crucial for reproducible and controlled production processes but also for ensuring the adequate biological response upon implantation in bone tissue. These materials have been considered as bioactive and osteoconductive as they bond directly to bone tissue without an interstitial fibrous tissue layer. Their bioactivity is related to the solubility of CaP in physiological media. Several groups have shown that biphasic CaP bioceramics composed of HA and β-TCP represented the optimal formulation in terms of bioactivity. The TCP phase is soluble and leads to a release of calcium and phosphate ions which saturate local body fluids and precipitate a biological apatite onto the less soluble HA crystals [6, 12, 13].
4.2.2 Injectable Calcium Phosphate Bioceramics and Putties
CaP bioceramic granules associated with hydrosoluble polymers are at present extensively used [14]. Different combinations have been proposed with polymers such as gelatine, collagen, fibrin, demineralized bone matrix, hyaluronic acid or synthetic polymers such as poloxamer, and cellulose derivatives. To date, several injectable biomaterials have been developed. Some of these injectable bone substitutes are made of CaP hydraulic cements that harden in the bone defect [15–17]. Others are composed of CaP granules suspended in hydrogel, as they are the most interesting carriers used for the development of injectable bone substitute.
MBCP Gel is a non-self-hardening injectable biomaterial. It is composed of BCP granules associated with a hydrosoluble polymer. These materials have been shown to be perfectly biocompatible and potentially resorbable, and thanks to their initial plasticity, they assume the shape of bone defects very easily, eliminating the need to shape the material to adjust it to the implantation site [14, 18]. MBCP Gel does not have the mechanical properties of hydraulic bone cements. However, bone cells are able to invade the spaces created by the disappearance of the polymer carrier. Bone ingrowth takes place all around the granules at the expense of the resorption of the BCP granules (Fig. 4.1). In time, the mechanical properties are increased due to the presence of the newly formed bone. Numerous reports both in vitro and in vivo have confirmed the efficacy and performance of this concept for an injectable bone substitute used in bone reconstruction [14, 18–20].

Fig. 4.1
MBCP Gel (In’Oss™). Human bone regeneration after revision surgery of nonintegrated dental implant, 8 months. SEM showing in grey the newly formed bone and in light grey the residual bioceramics particles integrated in the regenerated bone
IBS2 [21, 22] is a self-hardening composite. The BCP granules are associated with silanized HPMC-Si hydrogel. The guiding principles of silanized HPMC-Si hydrogel are its hydrophilic and liquid properties (it is viscous before being mixed with the CaP load and injection) and its pH-controlled reticulation process [23, 24]. The silanized hydrogel/CaP composite presents self-reticulation properties, due to the change in pH as a catalyst and without an exothermic effect. Once in the implantation site, in contact with the biological buffer liquids, a chemical reaction without additive and without any catalyst allows bridging and reticulation between the various macromolecular chains.
Prior to cross-linking, the composite is an injectable viscous liquid that hardens in the bone defect forming a gel loaded with BCP ceramic particles. IBS2 can entirely fill and remain in the bone defects. The BCP particles provide bioactivity supporting the bone healing process by osteoconduction. The cross-linked HPMC-Si hydrogel provides intergranular spaces for bone ingrowth. However, the jellification before blood diffusion delays osteoconduction of the BCP particles, and cells and tissue colonization at the expense of the composite.
Jellification is an important property of this material. It prevents the material from being washed out from the bleeding transplant site, after implantation. For an injectable bone substitute to maintain the bioceramic granules in unclosed cavities, the reticulation must increase the density of the material, reduce the dissolution or degradation of the polymer, and delay diffusion of the biological fluid and cell colonization [24].
The advantage of ready-to-use mixtures is their easiness of use and the reproducibility of the final material. Their kinetics for osseous reconstruction can be fast because of the many intergranular paths. These materials have relatively few intrinsic primary mechanical properties, even if the vehicles used harden by reticulation. Achieving mechanical properties is secondary to rapid physiological bone ingrowth.
BCP/fibrin glue: The association of bioceramics (TricOs®) and fibrin sealants may be interesting for the clinical applications of composite bone substitutes [25, 26]. Indeed, CaP granules are not easy to handle, are limited to filling bone cavities, and are not available for bone apposition. In addition, adding bioactive factors can improve the performance of bioceramics. In this regard, the adjunction of a binding agent, such as fibrin glue, improves the stability of the granules at the site of implantation and provides the scaffold effect of bioceramics with the additional osteogenic property. Fibrin-CaP composite could be obtained by mixing Baxter’s fibrinogen, the thrombin components of fibrin sealant (Tisseel® Baxter BioSciences BioSurgery), and TricOs® granules [27]. Macroporous Biphasic Calcium Phosphate TricOs® is a mixture of HA/β-TCP in a 60:40 ratio. Granules of 1–2 mm in diameter presenting both macroporosity (50–55 %) and microporosity (30–35 %) are used. To enhance the working time, a low thrombin concentration (4 U) is used. The Tisseel/TricOs volume ratio is 1 to 2. Numerous preclinical studies have been performed in rabbits and goats, both for biocompatibility and biofunctionality, using, for example, sinus lift augmentation and bone filling in long bone. Histology, histomorphometry, and X-ray microtomography have demonstrated the osteogenic properties of the composite [27].
Calcium phosphate cements: The need for a material for minimally invasive surgery (MIS) prompted the development of a concept for self-setting injectable CaP cement (CPC) as bone substitute. Currently, several CPCs are commercially available and more are being investigated. LeGeros et al. first introduced the concept in 1982 [15], and the first patent was obtained by Brown and Chow in 1986 [16]. All current CPCs are reported to have good mechanical properties and reasonable setting times. However, after setting, these materials remain dense and do not provide rapid bone substitution because of the lack of macroporosity. Numerous studies have reported the applications of currently available commercial CPCs [1, 17]. New BCP-based CPCs have recently been developed [28]. The MCPC consists of multiphasic CaP phases, including BCP. In vivo, the components of the cement resorb at different rates, allowing the formation of interconnecting macroporosity, thus facilitating bone ingrowth and substitution of the cement with the newly forming bone [28].
The powder component is essentially made of a settable and resorbable matrix (which includes α-TCP, stabilized amorphous CaP (s-ACP), and monocalcium phosphate monohydrate (MCPM)). A sieved fraction of macroporous BCP granules ranging between 80 and 200 μm in diameter are incorporated into the matrix. The cement liquid is an aqueous solution of Na2HPO4.
After setting MCPC in distilled water at 20 °C, the mechanical properties in compression of such materials were 10 ± 2 MPa at 24 h and 15 ± 2 MPa after 48 h. The cohesion time for injectability was reached after 20 min. Animal models of critical size defects in rabbit epiphyses or goat vertebral bodies demonstrated the performance and efficacy of CPC. MBCP granules act as a scaffold for bone osteoconduction, and resorption of the ACP content of the cement allowed macroporosity and bone ingrowth between and at the surface of the BCP granules, extending to the core of the implanted site. The cement matrix dissolved as expected, forming an open structure for cell colonization and bone ingrowth at the expense of the self-setting bone void filler [7, 28].
4.2.3 Fundamental Physicochemical Properties
4.2.3.1 Resorption
CaP bioceramic resorption [29] is the process by which resorption cells such as macrophages and osteoclasts break down the biomaterials and release the ions, resulting in a transfer of calcium and phosphate from the bioceramic to the blood [30]. This active process undertaken by the cells is equivalent to bone remodeling. The process is largely associated with the degradation of the bioceramic [6, 31] by biological fluids, the mechanical stress of the implantation site, releasing smaller particles. In the inflammation process, the size of the particles has a strong influence on dissolution [32], leading to the conclusion that resorption is dependent on the dissolution.
4.2.3.2 Dissolution
The solubility of CaP phases is mainly related to their chemical composition and crystal properties [3, 6, 29, 33, 34]. Different solubility product constants (K sp) have been reported for synthetic or biological CaP compounds, as shown in Table 4.1 [6]. The solubility is affected by cationic or anionic substitutions in the apatite lattice. For instance, carbonated or CDA are more soluble than fluoroapatite (FAP). Comparative dissolution in acetate buffer provided the following order of solubility: bone >> enamel >> β-TCP > HA. β-TCP has been found to dissolve faster in physiological solutions, as well as exhibiting a greater rate of dissolution or degradation than HA, when implanted in heterotopic or ectopic sites. Depending on the HA to β-TCP weight ratios, the solubility of BCP ceramics will be more similar to that of β-TCP or HA. The dissolution of CaP ceramics is also affected by its porosity and particle size. Increasing the porosity greatly enhances the surface area in contact with fluids and, thus, leads to a faster dissolution rate. As shown in Fig. 4.2, CaP ceramics exhibit macropores with diameter sizes ranging from 200 to 600 μm. While macropores are well interconnected, they permit the percolation of fluids, cells, and tissues within their structure. As illustrated in Fig. 4.2b, some ceramics may also exhibit a microporous surface. Spherical CaP grains appear bounded by necks leaving tiny pores approximately 0.1–1 μm in size. This remaining microporosity results from incomplete sintering of ceramics, especially when poorly crystalline precursor CaP powders and low sintering temperature and time (e.g., 1,000–1,200 °C for 1–10 h) are used. The lower temperature of sintering has a significant role in the formation in lattice defect. The lattice defect is particularly involved in the process of dissolution [32] explaining the large difference in the solubility of different HA scaffolds. According to the crystal size, lattice defect, and active cell resorption, the entire CaP can be resorbable. Only the kinetics differs according to time. A small-sized HA crystal with lattice defect is more resorbable than large, high crystalline TCP for example.
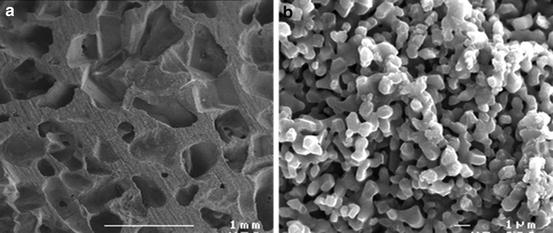

Fig. 4.2
(a) MBCP+, Micro- and Macroporous Biphasic Calcium Phosphate, macropores. (b) MBCP+, Micro- and Macroporous Biphasic Calcium Phosphate, micropores
4.2.3.3 Absorption
The degradation and dissolution processes release high levels of calcium and phosphate ions into the extracellular fluid as the osteoclasts tunnel into the mineralized bone. Contrarily to degradation and elimination of some biomaterials, CaP ceramics elements released are absorbed during the physiopathological processes during bone regeneration. The released Ca and PO4 ions are precipitated at the surface of the residual crystals, and secondary nucleation and hetero epitaxic growing processes have been reported [11, 13]. This process has also been observed in Bioglass by Larry Hench [33], and the basis of bioactivity has been well documented by Kokubo using simulated body fluid incubation [34].
4.2.4 Fundamental Biological Properties
4.2.4.1 Osteointegration and Osteocoalescence
All CaP ceramics have been found to be biocompatible. HA ceramics are considered as non-resorbable, while β-TCP is resorbable based on the amount of implant left as a function of time. It is now generally accepted that CaP ceramics are bioactive and osteoconductive. Bioactivity is a property of the ceramic surface that induces biological integration of soft and hard living tissues. The core mechanism of bioactivity is the partial dissolution and release of ionic products in vivo, elevating local concentrations of calcium and phosphate and precipitating a biological apatite on the surface of the ceramics. This process of dissolution/precipitation has been studied in detail using transmission electron microscopy (TEM) of ceramics implanted in ectopic or heterotopic sites. The BCP grains partly dissolve in body fluids leading to the precipitation of tiny apatite crystals on their surface or between the grains. TEM studies have shown that these apatite crystals were similar to bone apatite in size, shape, and electron diffraction patterns [11]. The abundance of the apatite microcrystals associated with large grains of ceramics appeared to be directly related to the HA to β-TCP ratio of BCP ceramic implants. A higher amount of β-TCP gave a greater amount of precipitated apatite crystals. These microcrystals were identified as carbonate-containing apatites that are associated with an organic matrix similar to bone.
This surface precipitation may incorporate various proteins and growth factors present in the microenvironment, which may subsequently promote cell attachment and function. The bioactive ceramics are assumed to have a surface phase biologically equivalent to bone mineral. It suggests that osteoblasts are attracted to this layer and produce bone extracellular matrix leading to bone apposition (osteoconduction), rather than fibrous tissue encapsulation of ceramics. This “bone bonding” has also been called osseocoalescence [35], a better definition to explain the chemical bonding between CaP bioceramics and bony crystals.
4.2.4.2 Osteoconduction
In 1991, Damien described osteoconduction as the ability of a substitute (graft or material) to promote development of vascular and osteoprogenitor cells from the recipient site [36]. In 1998, Davies described osteoconduction as the process by which the bone is directed into the material structure, pore channels, from the surface of materials by an invasion of differentiated oestogenic cells [37]. This specific integration of the surface of a substitute in direct contact with the bone, without the interposition of fibrous tissue, is called osteogenesis link [37]. In 1999, Cornell defined osteoconduction as the passive ability of a material to promote bone growth, and which supports continuity or cellular and vascular invasion [38]. In 2001, Albrektsson described osteoconduction as bone growth on a surface. An osteoconductive surface allows bone ingrowth into the pore channels and on its surface [39].
4.2.4.3 Osteogenicity and Osteoinduction
Osteoinductivity is the ability of a material to induce osteogenesis and is demonstrated by bone formation in non-osseous (heterotopic) sites. Osteoinductive factors have previously been associated only with bone morphogenetic proteins (BMPs) present in bone matrix. However, many studies have reported osteoinductive properties associated with biomaterials. Several factors that contribute to the osteoinductive property of a material include the animal model, implantation sites, and biomaterial properties [40–44]. Biomaterial properties include composition, surface roughness, and geometry (concavities, porosities). To improve the biological properties of biomaterials and bone substitute in order to optimize the healing of bone critical size defects, it is necessary to evaluate their ability to induce osteogenesis in ectopic sites and to have a complete physicochemical characterization. For example, the specific surface area of the bioceramics crystals is generally not evaluated or reported in the articles in relation with CaP scaffolds associated with MSCs, as reported by Gibson [8]. The lack of information, the controversial studies, and the semantic aspect of osteogenicity or osteoinduction hamper the correct evaluation of intrinsic osteoinductive properties of CaP bioceramics.
In 1889, Senn reported that the implantation of decalcified dog bone promoted healing of large-bone defects [45], but the work was difficult to reproduce [46]. In 1931, Huggins observed an ectopic ossification after implantation of soft tissue (bladder wall) in the superficial muscle of the abdomen (rectus abdominis) of adult dogs [47]. He found an osteoid tissue 16 days after implantation, but did not find a mineralized bone tissue. From day 18 to 26 post implantation, he observed a characteristic bone tissue (tissue and cell components), formed by intramembranous ossification. This type of structure is generally present in the bones of the skull, shoulder blades, and the ilium [48]. During the past 30 years, Levander found ossification in muscle tissue after injection of extract of pure alcohol [49, 50]. In 1958, Bridges observed formation of bone and cartilage in nonskeletal tissue (kidney capsule, subcutaneous) after implantation of devitalized tissue or tissue extracts (epiphyseal cartilage, bone fragments) [51]. Urist and McLean in 1952, training in ectopic anterior chamber of the eye, described ectopic bone formation [52]. Urist described induced bone formation in ectopic sites using decalcified bone matrix in several species [53].
In 1968, Friedenstein defined osteoinduction as the “induction of undifferentiated inducible osteoprogenitor cells that are not yet committed to the osteogenic lineage to form osteoprogenitor cells” [54]. In 1987, Wilson-Hench described osteoinduction as the process by which osteogenesis is induced [55
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


