Table 1.1 • CL±P loci.
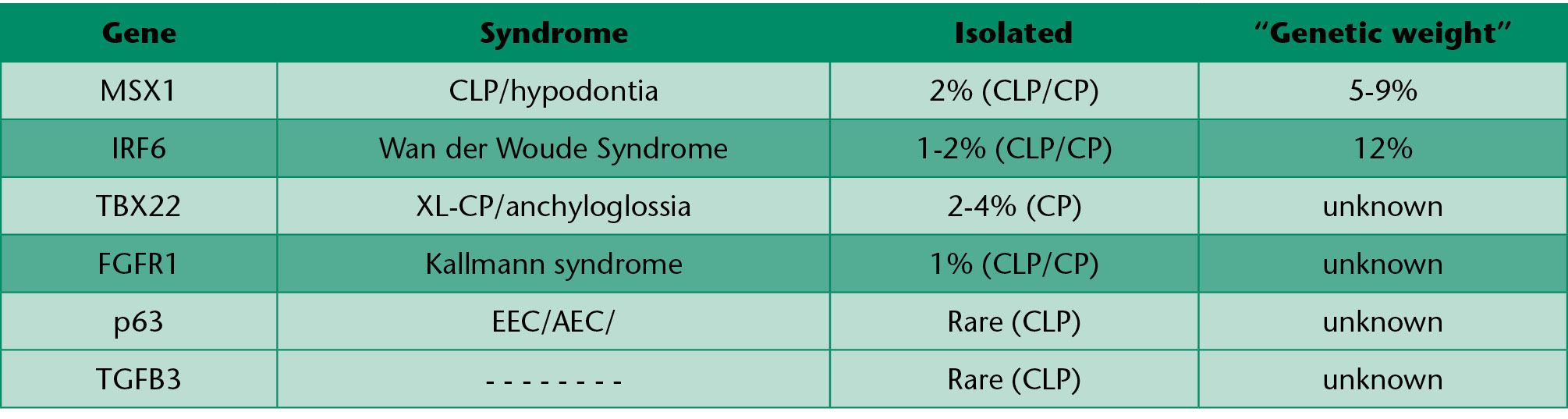
Table 1.2
MSX 1
MSX 1 gene variants have been found to make the embryo susceptible to maternal smoke, as recently demonstrated by van den Boogaard et al (2008), Chevrier et al (2008).
The result of the first study, involving on the whole 412 cases and 399 controls, showed that preconception smoke of both parents interacted with a specific allelic variant of MSX 1 significantly increasing the risk of cleft lip and palate of their children.
The second case-control study, including 240 non-syndromic cases and 236 controls, suggested an additional interaction, especially between ETS and CYP 1 A1 or MSX 1 and maternal smoke and CYP 2E1.
Mutations of MSX 1 have also been identified in about 2% of the non-syndromic cases of cleft lip and palate (Jezawski, 2003).
IR F6
Interferon regulatory factor 6 (IR F6) belongs to the family of the transcription factors which regulate the expression of alfa and beta interferon induced by a viral infection.
Mutations on IRS6 are the cause of some syndromic conditions such as the van der Woude syndrome and the popliteal pterigia.
The van der Woude syndrome is a condition with a mendelian transmission which is characterized by cleft lip and/or cleft palate, and represents around 2% of all the cases of familial cleft lip and palate.
Syndromic cleft lip and palate
As previously mentioned, there are more than 400 syndromic conditions in which one of the symptoms is represented by cleft lip and palate with and without cleft palate. The following are some of the most important syndromic conditions associated with cleft palate.
Van der Woude syndrome (CLP and CP)
It is a defect of the craniofacial development, characterized by the Association of inferior labial pits and cleft lip and/or cleft palate. It is the most frequent syndrome within the syndromic cleft lip and palate patients. The clinical presentation is extremely variable, also within the same family, and includes all possible combinations: isolated labial pits of the inferior lip, or oligodontia, or cleft lip or isolated cleft palate of variable severity. The most frequent signs are the labial pits, which are present in 80% of the patients. The transmission is autosomal dominant with high penetrance (80-97%).
Microdeletion 22q11.2 (CP) syndrome
It is a craniofacial anomaly associated with thymus and parathyroid hypoplasia, congenital heart disease, and mild facial dysmorphism.
Besides the tronco conal congenital heart disease, cleft palate or velar insufficiency, facial dysmorphism and learning disorders may also be found.
The Micro deletion 22q 11 can be transmitted as a dominant character being one parent carrier of the same deletion in 10-20%.
Stickler syndrome (CP)
Stickler syndrome is a genetic condition characterized by ocular symptoms, Pierre Robin sequence (retrognatia, glossoptosis, horseshoe cleft of the soft and hard palate), skeletal deformities and neurological deafness (10% of the cases).
Treacher Collins syndrome (CP)
Treacher Collins syndrome is a genetically dominant syndrome with a penetrance of 90%, a variable expression, and an incidence of about 1 in 50,000 births.
It is associated to auricular hypoplasia (77%), atresia of the external auditory canal (36%), anomalies of the ossicular chain, conductive deafness (40%), hypoplasia of the zygomas (80%), with down-slanting palpebral fissures, inferior eyelid coloboma (69%), absence of the eyelashes on the lateral third of the inferior eyelid, mandibular hypoplasia (70%), palatal cleft (20%). Facial deformities are bilateral and symmetrical. It is generally due to a mutation of the gene TC 0F1.
Opiz syndrome (CLP)
This rare syndrome is characterized by craniofacial anomalies (hypertelorism with telecanthus, wide nasal bridge and occasionally cleft lip and palate), uro-genital anomalies, laryngeal or coaxial anomalies, and less frequently, other anomalies of the midline.
We may distinguish two forms of Opiz syndrome G/BBB: one with an autosomal dominant transmission and the other X linked.
The mutations of the gene M ID 1 (which encodes for the protein 1 of the midline) explains 80% of the X linked form.
Kabuki make-up syndrome
This condition presents with facial dysmorphism including inferior eyelid eversion, long palpebral fissures accentuated curve of the eyebrows, long and thick eyelashes, blue sclera, flattened tip of the nose, cleft lip and palate or narrow palate, prominent deformed ears with preauricular fistulae and dental anomalies.
About 50% of the children with Kabuki make-up syndrome have a congenital heart disease.
The children may present, as additional features, mental retardation (mild to moderate) and postnatal growth retardation.
The vast majority of the cases are sporadic, but rare familial cases have been observed, which suggest possible dominant transmission with variable expression.
Smith Lemli Opiz syndrome (CP)
This rare syndrome is characterized by facial dysmorphism such as micrognathia or retrognathia, low set ears, philter anomalies and cleft palate, microcephaly (100%). Growth retardation is a constant feature because of feeding disorders.
Mental retardation is always severe. Other signs are: incomplete development of the male genitalia, syndactily of the second and third fingers of the foot (constant). This condition is transmitted as autosomal recessive trait, caused by the mutation of the gene that codes for the 7D Hydro cholesterol reductase enzyme, which is localized on chromosome 11q13.
X linked cleft palate and anchyloglossia
Braybrook et al. (2001) have demonstrated that X linked cleft palate associated with anchyloglossia, may be caused by a mutation of the G TB X 22. This condition is located on chromosome Xq21.3. Inheritance is generally X-linked recessive, but some mothers of affected children show anchyloglossia as well. Cases with cleft palate only or with anchyloglossia only, or both symptoms have been reported.
EEC syndrome (CLP)
It is a syndrome characterized by the association of limbs anomalies such as cutaneous and/or bony syndactily or ectrodactily, ectodermal dysplasia and cleft lip and palate. It is characterized by an intra and inter-familial variable expression with mild and severe forms. EEC syndrome is due to a mutation of the P63 gene and it is transmitted as an autosomal dominant trait.
Recurrence risk
Isolated cleft lip and palate
The aetiology of the isolated cleft palate, when associated malformations and syndromes have been excluded, has a multifactiorial etiology with a combination of genetic and environmental factors (Table 1.3).
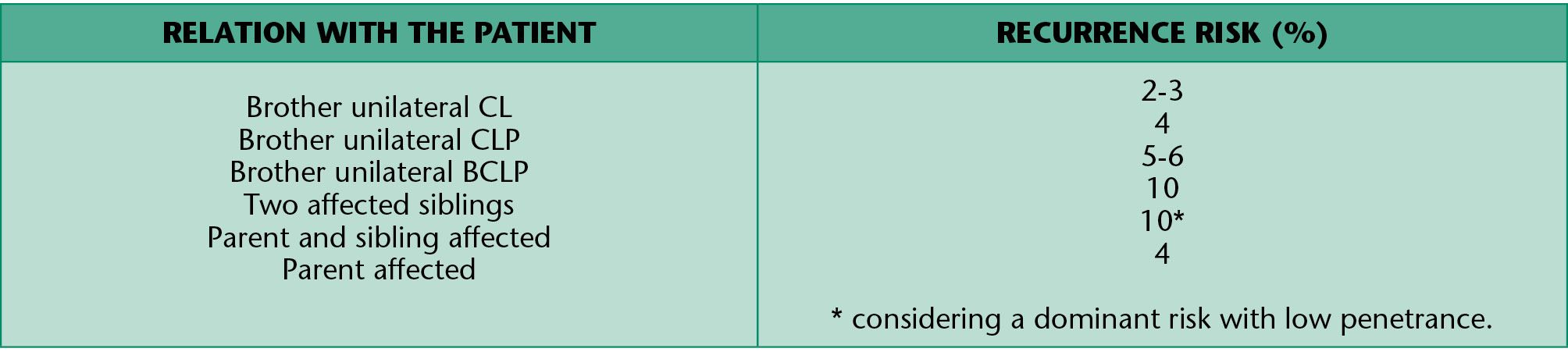
Table 1.3 • Recurrence risk for isolated CL and CLP.
Isolated cleft palate
Children with an isolated cleft palate have more commonly other associated anomalies or genetic syndromes (Table 1.4). The risk of recurrence of cleft palate is summarized in table 1.4 and varies according to the etiology.
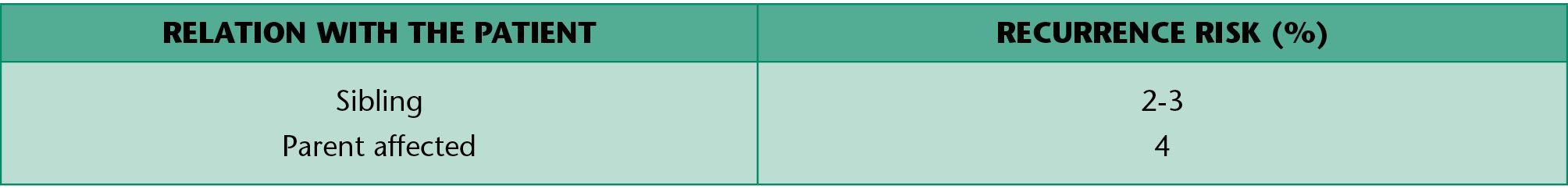
Table 1.4 • Recurrence risk for isolated CP.
REFERENCES – genetics
Braybrook C, Doudney K, Marcano AC, Arnason A, Bjornsson A, Patton MA, Goodfellow PJ, Moore GE, Stanier P. The T-box transcription factor gene TBX22 is mutated in X-linked cleft palate and ankyloglossia. Nat Genet. 2001; 29(2): 179-183.
Carinci F, Scapoli L, Palmieri A, Zollino I, Pezzetti F. Human genetic factors in nonsyndromic cleft lip and palate: an update. Int J Pediatr Otorhinolaryngol. 2007 Oct; 71(10): 1509-19. Epub 2007 Jul 2. Review.
Chevrier C, Bahuau M, Perret C, Iovannisci DM, Nelva A, Herman C, Vazquez MP, Francannet C, Robert-Gnansia E, Lammer EJ, Cordier S. Genetic susceptibilities in the association between maternal exposure to tobacco smoke and the risk of nonsyndromic oral cleft. Am J Med Genet A. 2008 Sep 15; 146A(18): 2396-406.
Ferrero GB, Baldassarre G, Panza E, Valenzise M, Pippucci T, Mussa A, Pepe E, Seri M, Silengo MC. A heritable cause of cleft lip and palate-Van der Woude syndrome caused by a novel IRF6 mutation. Review of the literature and of the differential diagnosis. Eur J Pediatr. 2009 Jun 18. [Epub ahead of print].
Jezewski PA, Vieira AR, Nishimura C, Ludwig B, Johnson M, O’Brien SE, Daack-Hirsch S, Schultz RE, Weber A, Nepomucena B, Romitti PA, Christensen K, Orioli IM, Castilla EE, Machida J, Natsume N, Murray JC. Complete sequencing shows a role for MSX1 in non-syndromic cleft lip and palate. J Med Genet. 2003 Jun; 40(6): 399-407.
Marazita ML, Lidral AC, Murray JC, Field LL, Maher BS, Goldstein McHenry T, Cooper ME, Govil M, Daack-Hirsch S, Riley B, Jugessur A, Felix T, Morene L, Mansilla MA, Vieira AR, Doheny K, Pugh E, Valencia-Ramirez C, Arcos-Burgos M. Genome scan, fine-mapping, and candidate gene analysis of non-syndromic cleft lip with or without cleft palate reveals phenotype-specific differences in linkage and association results. Hum Hered. 2009; 68(3): 151-70. Epub 2009 Jun 11.
Van den Boogaard MJ, de Costa D, Krapels IP, Liu F, van Duijn C, Sinke RJ, Lindhout D, Steegers-Theunissen RP. The MSX1 allele 4 homozygous child exposed to smoking at periconception is most sensitive in developing nonsyndromic orofacial clefts. Hum Genet. 2008 Dec; 124(5): 525-34. Epub 2008 Oct 19.
2. EMBRYOLOGICAL DEVELOPMENT of CLP and clinical features
(M.C. Meazzini)
Cleft lip and palate is determined by the lack of fusion of the fronto-nasal and maxillary prominences during the seventh week of gestation, to which may follow, as a secondary event in the following week, the lack of fusion of the palatal processes.
NORMAL EMBRYOLOGICAL DEVELOPMENT OF THE COMPONENTS INVOLVED WITH CLP
The most typical features in the development of the head and neck are the “branchial or pharyngeal arches” which appear during the fourth and fifth week of embryonic life. These arches are separated externally by the “branchial grooves” and internally by the “branchial pouches”. Each branchial arch consists of an axis of mesodermal tissue, covered externally by an ectodermal layer and internally by epithelium of endodermal origin.
In addition to the local mesenchyme, the arches receive a large number of neural crest cells that, migrating into these, contribute to the formation of the skeletal and muscular components of the face. The original mesoderm of the arches gives rise to the muscles of the face and neck. Each arch is therefore characterized by its own muscular and neuro-vascular components.
At the end of the 4th week of development the central part of the face is formed by the stomodeum surrounded by the first pair of pharyngeal arches. The stomodeum is the primitive oral cavity and appears during the 3rd week of embryonic life. This is initially a virtual cavity bounded by the forebrain superiorly, by the pericardial sac inferiorly and posteriorly by the bucco-pharyngeal membrane, which separates it from the cranial portion of the gastro-intestinal tract (pharynx). The bucco-pharyngeal membrane atrophies by the fourth week, thus creating continuity with the gastro-intestinal tract.
When the embryo is at its 4th week of development, five mesenchymal processes can be found: two mandibular processes (1st pharyngeal arches) that are visible caudal to the stomodeum, two maxillary processes (dorsal side of the 1st pharyngeal arch), located laterally to the stomodeum, and the frontal prominence, a small swelling positioned cranially to the stomodeum. On each side of the frontal process, and precisely above the stomodeum, there is a small thickening of the surface ectoderm, the nasal placode. During the 5th week two ridges are formed. The internal and the external nasal processes, which surround the nasal placode which is transformed into the floor of a depression called the nasal cavity.
During the following two weeks the maxillary processes grow medially and continue to push the medial nasal processes toward the midline, so that they fuse together and at the same time merge laterally with the maxillary processes (Figg. 2.1-2.1a).
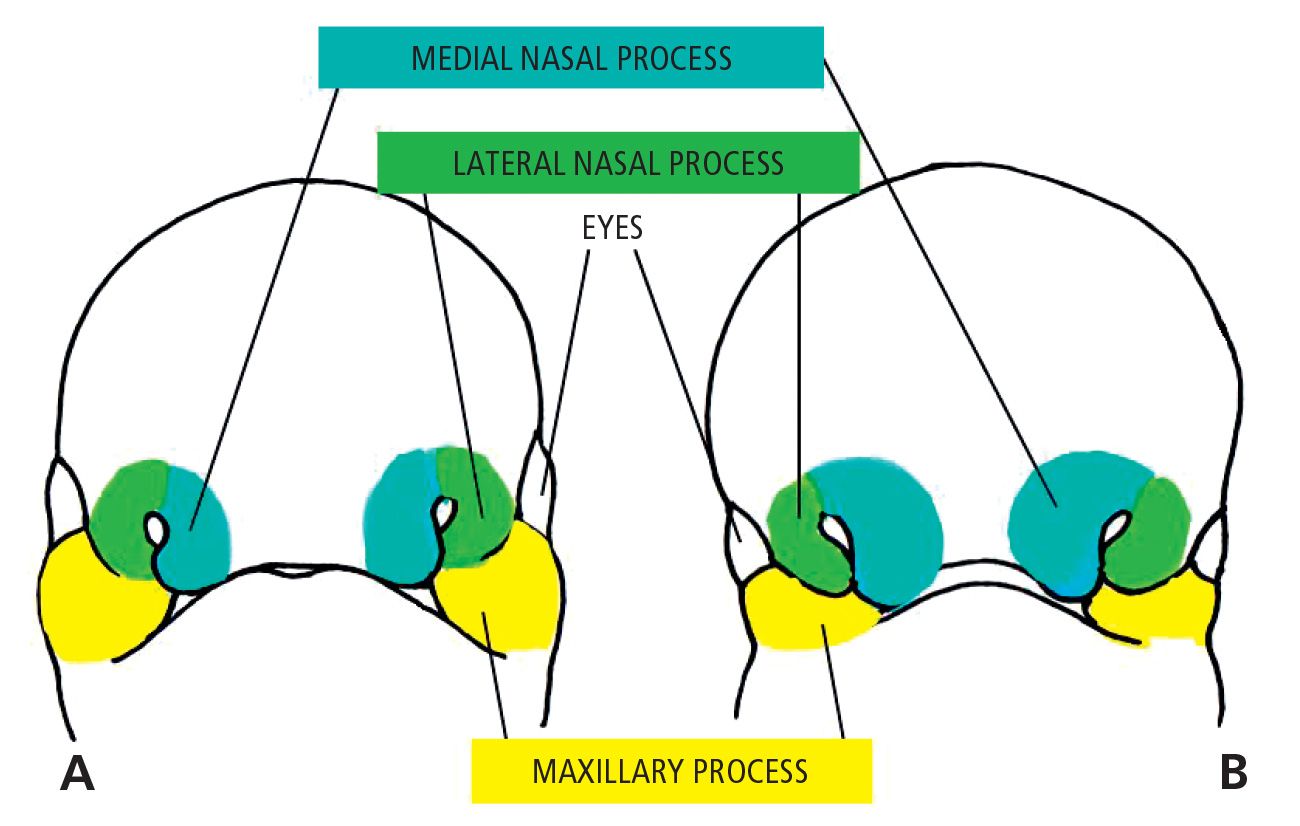
Fig. 2.1 • A. 5 week embryo. B. 6 week embryo.
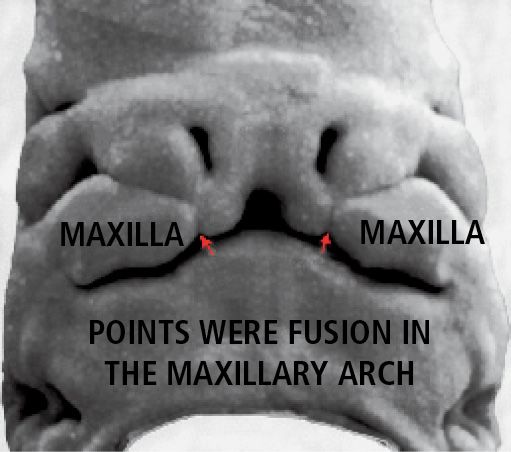
Fig. 2.1bis • 6 week embryo.
The upper lip is, therefore, formed by the fusion of the two medial nasal processes and the two maxillary processes. The lateral nasal processes do not contribute to the formation of the upper lip, but form the nasal alae (Fig. 2.2).
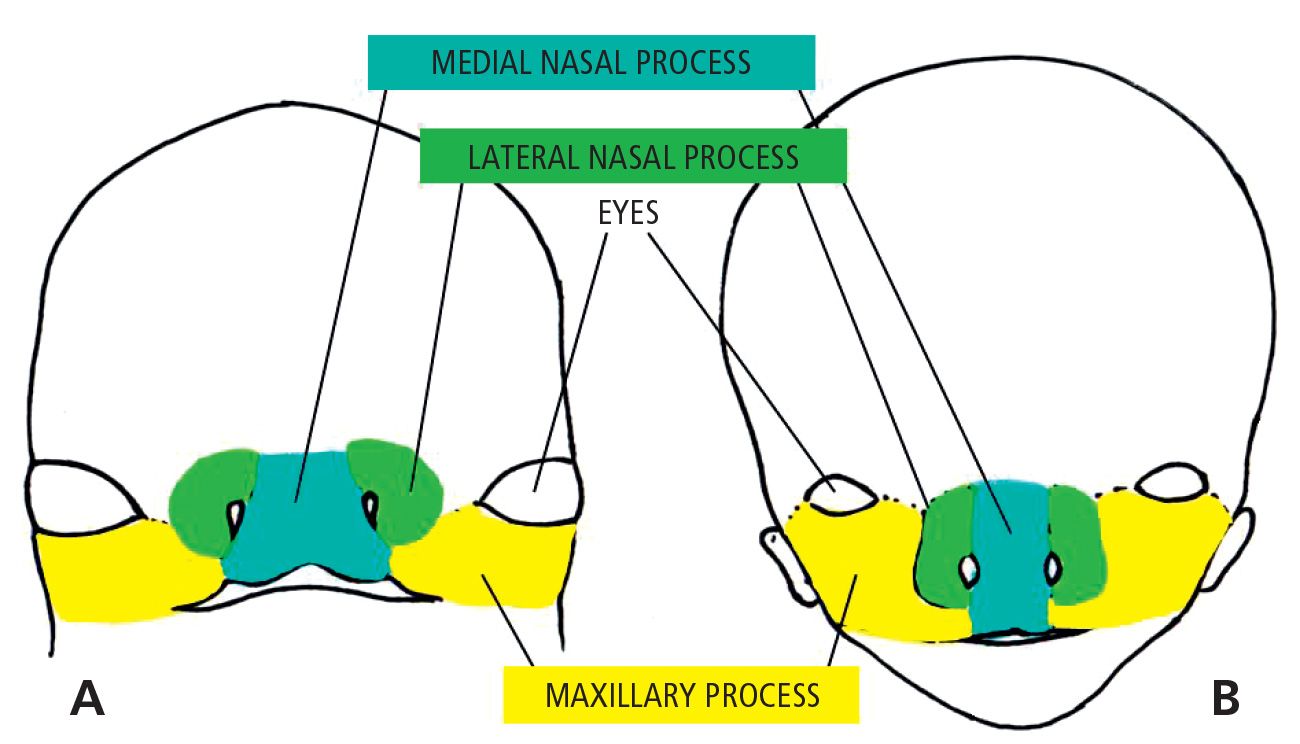
Fig. 2.2 • A. 7 week embryo. B. 10 week embryo.
As a result of the medial growth of the two maxillary processes, the two medial nasal processes fuse. The structures that are formed constitute the intermaxillary segment, comprising: a labial component, that forms the philtrum and the tubercle of the upper lip, the premaxilla and the primary palate.
A small portion of the inside of the nose comes from the intermaxillary segment as well, and forms the columella and the tip of the nose. Cranially the maxillary segment is continuous with the rostral portion of the nasal septum, formed by the frontal process.
During the 6th week of gestation, fusion of the lip occurs, while the processes internally directed, the palatal lateral laminae, develop from the maxillary processes that surround the stomodeum. At the same time the growth of the tongue proceeds very quickly, so that it completely fills the oro-nasal cavity around the 7th week (Fig. 2.3).
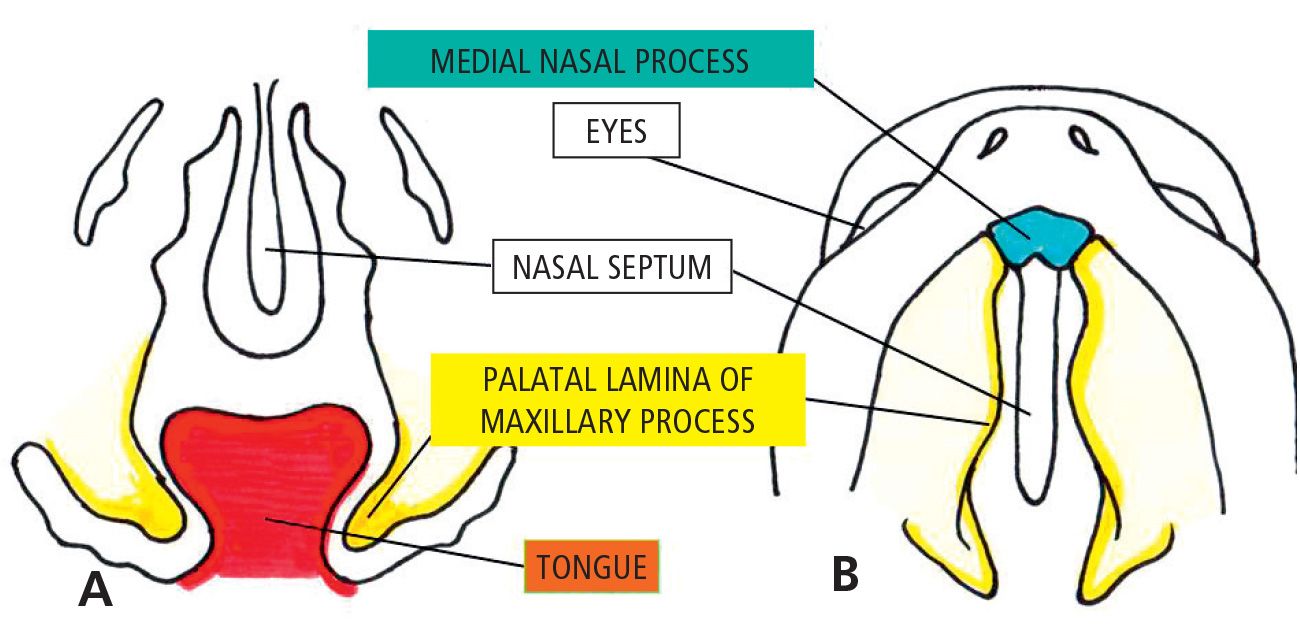
Fig. 2.3 • 6 1/2 week embryo. Note the palatine processes are still growing downwards with the tongue interposed.
The growth of the chamber of the stomodeum during the 8th week of development allows the tongue to fall in the lower part of the cavity and free the palatal shelves (Fig. 2.4).
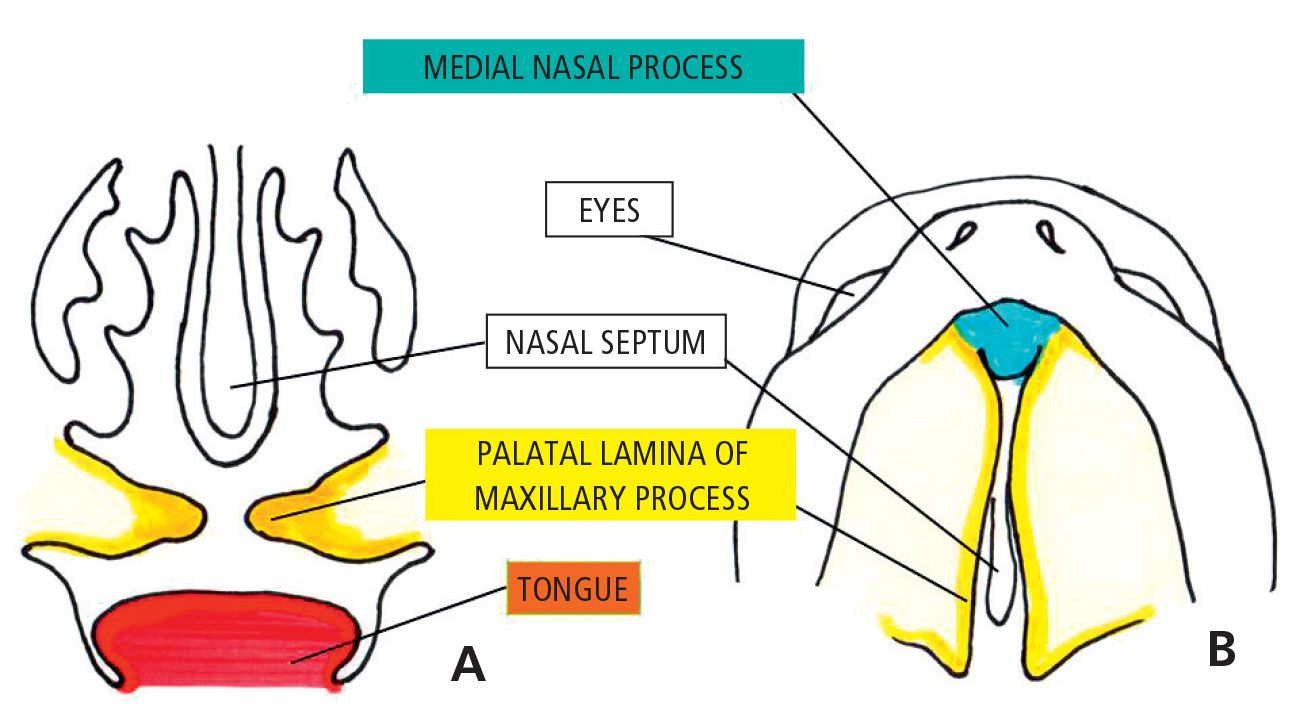
Fig. 2.4 • 7 1/2 week embryo. Note the palatine processes growing horizontally one towards the other.
These are then able to slide on a horizontal plane, whereby contacting the septum on the midline and, more anteriorly, with the primary palate. Thereafter, the free ends of the palatal shelves meet and the contact of the epithelial lining the shelves stimulates a process of “apoptosis”, programmed cellular death, which allows for the processes to fuse.
The fusion begins during the 8th week and ends in the 12th week (Fig. 2.5).
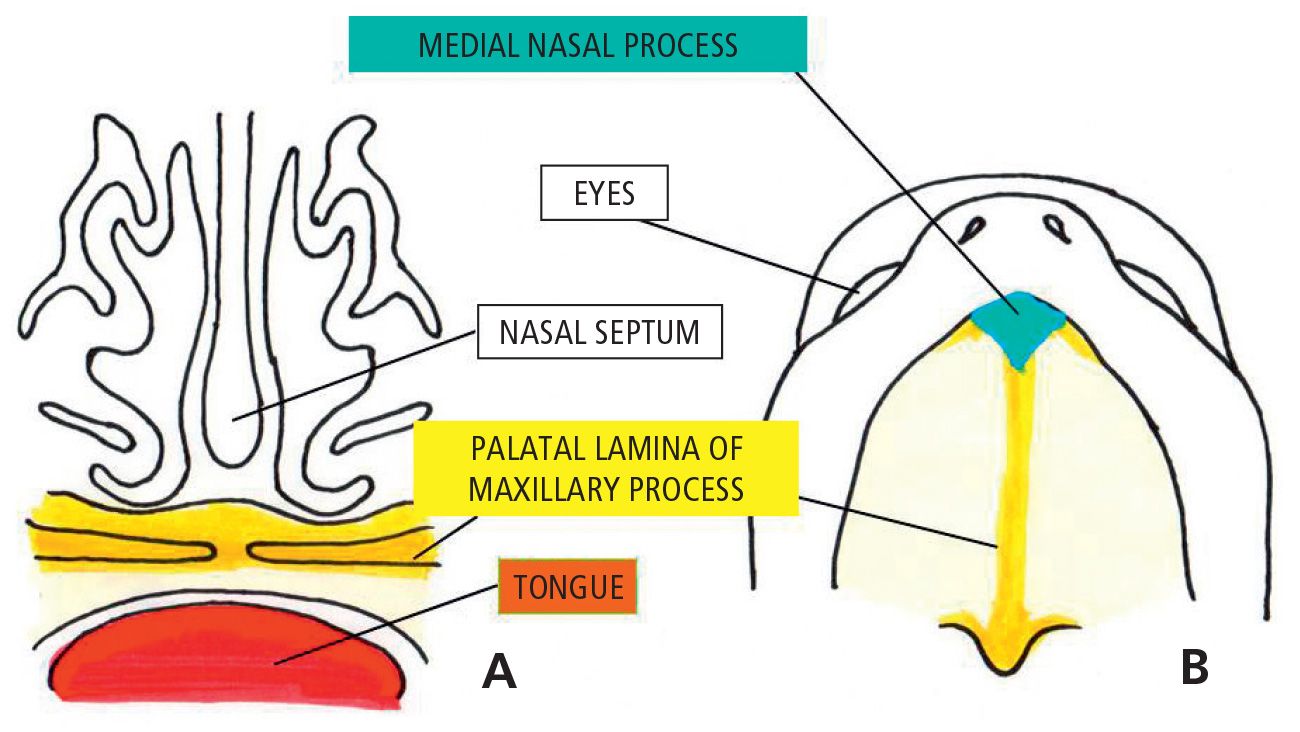
Fig. 2.5 • 10 week embryo. Note the palatine processes are fused.
The passage from a vertical to a horizontal position of the palatal plates is accomplished in a few hours, beginning earlier in the male than in female embryos: this difference perhaps accounts for the different incidence of cleft palate in both sexes.
Merging, the two palatal processes, form the secondary palate. Anteriorly the fusion takes place with the triangular primary palate, so that the incisal foramen indicates the boundary on the midline between primary and secondary palate.
At the same time of the fusion of the palatal plates, the septum grows caudally and fuses with the cephalic side of the palate, separating the oral cavity from nasal and dividing the latter into two nasal cavities. (Burdi, 1969).
CLINICAL CLASSIFICATION of CLEFTS
(Maue-Dickson, 1979)
There are very different forms of cleft lip and palate and of varying severity, which can be divided into two groups based on etiology and morphology. We may divide clefts of the lip and alveolus, with or without cleft palate, from isolated cleft palate, which arises at a later stage, after the normal development of the lip and jaw. In general, clefts may be be uni- or bilateral, as well as total or partial.
ISOLATED CLEFT LIP
The cleft lip appears at the philtral edge and may be either incomplete or complete. The unilateral incomplete cleft lip may appear as a partial unilateral incisions of the vermilion border and lip (Fig. 2.6).
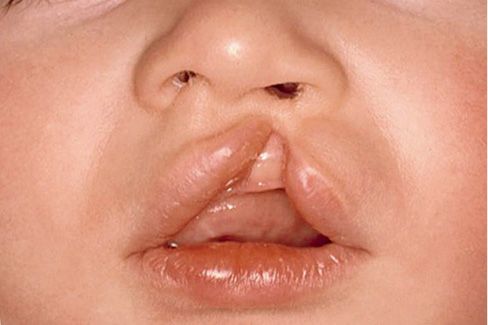
Fig. 2.6 • Unilateral incomplete cleft lip.
Depending on the extent, there may be a lateral deviation of the nasal ala. Sometimes notching of the alveolar ridge may be associated.
In the complete cleft lip there is a unilateral clefting of the lip up to the vestibule of the nose: In this case, therefore, the ala of the nose is distorted.
Sometimes the presence of skin bridges of different thickness in the large region of the nasal vestibule (the Simonart Band) simulates an incomplete cleft lip (Gundlach and Pfeiffer, 1979).
CLEFT OF THE LIP AND ALVEOLUS
(Fig. 2.7)
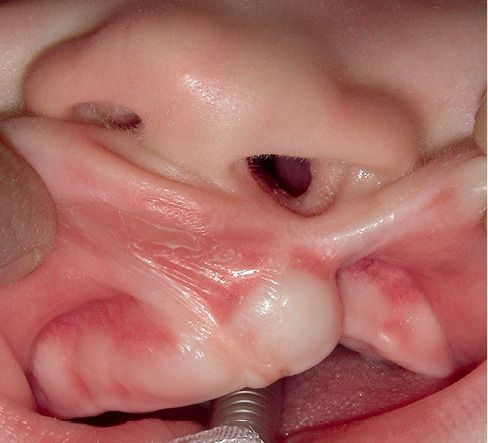
Fig. 2.7 • Unilateral cleft of the lip and alveolus.
Complete unilateral and bilateral clefts of the primary palate, cleft lip and alveolar clefts, are relatively rare. The alveolar process is usually significantly deformed and is separated until the incisal foramen, therefore, although the palate is intact, the entire anterior aspect of the nasal floor is missing.
In the unilateral cleft lip and alveolus, there is often a modification of the nasal shape and the nasal septum is deviated towards the non affected side.
As a result, the ala on the side of the cleft is flattened.
In bilateral forms, the pre-maxillary segment is isolated and secured only to the nasal septum and vomer.
COMPLETE CLEFT LIP AND PALATE
In complete unilateral cleft lip and palate the lips, jaw and palate are clefted. On the cleft side the entire bony part of the floor of the nose is lacking (Figs. 2.8a-b).
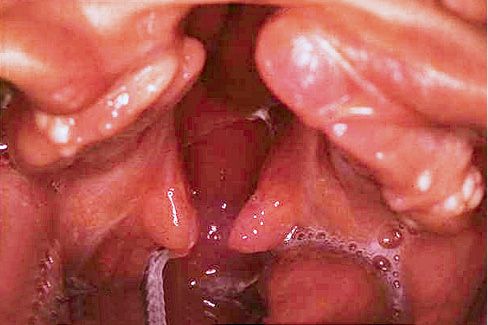
Fig. 2.8a • Intraoral Frontal view of complete unilateral cleft lip and palate.
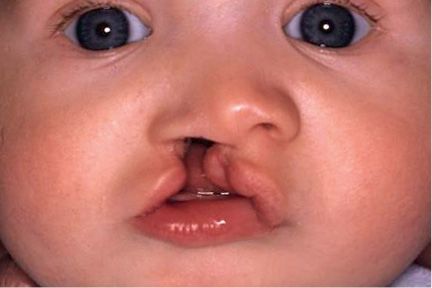
Fig. 2.8b • Extraoral frontal view of a complete unilateral cleft lip and palate.
The nasal septum is deviated, together with the vomer in the direction of the non cleft side, which alters the symmetry of the midfacial region.
In complete bilateral cleft lip and palate, which are among the most severe facial deformities, on both sides the entire bony structure of the nasal floor is missing. The premaxilla is isolated, connected to the vomer and the nasal septum,and may be extremely mobile. Sometimes it is well inserted into the alveolar process; in most cases, however, it is protruded (Fig. 2.8 c).
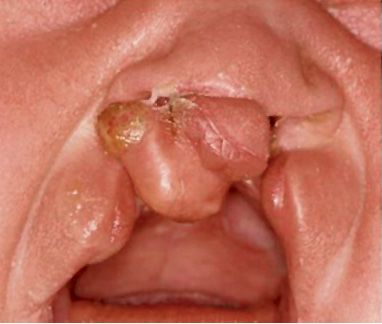
Fig. 2.8c • Extraoral frontal view of a complete bilateral cleft lip and palate with a rotated premaxilla.
Often the columella of the nose is absent and the tip of the nose very flat (Fig. 2.8d).
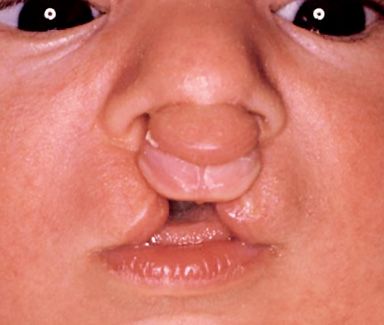
Fig. 2.8d • Extraoral frontal view of a complete bilateral cleft lip and palate with the absence of the columella.
The shape of the nose is almost always deformed. The dimensions of the premaxilla vary depending on the number of tooth buds for the incisal teeth which it contains. Combinations of complete and incomplete cleft lip and palate are not unusual.
ISOLATED CLEFT PALATE
The malformation affects the palate and can include both the hard and the soft palate, or the soft palate only (Fig. 2.9).
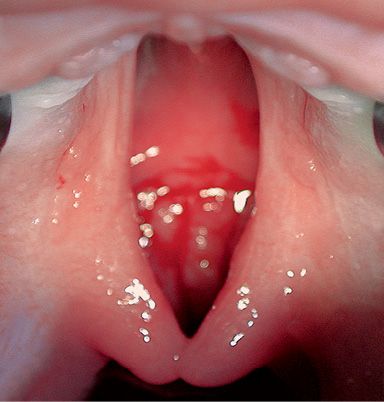
Fig. 2.9 • Intraoral occlusal view of an isolated cleft palate.
A cleft of the hard palate can begin at the nasopalatine duct or more posteriorly, affecting all, or only part, of the hard palate on the midline and it is always associated to a cleft of the soft palate.
In the Pierre-Robin sequence, characterized by cleft palate, microgenia and glossoptosis, the cleft palate usually is particularly wide, horseshoe shaped, with an impingement of the of the tip of the tongue which lodges into the nasal cavity.
CLEFT OF THE SOFT PALATE
In clefts of the soft palate the defect is limited to the muscles of the soft palate (Fig. 2.10).
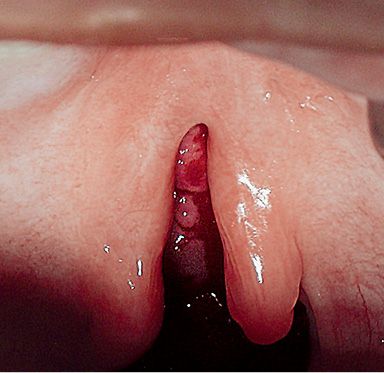
Fig. 2.10 • Intraoral view of a cleft of the soft palate.
The complete clefts of the soft palate start at the rear edge of the hard palate. The incomplete involve only the soft palate and the uvula. Sometimes only the uvula (bifid uvula) is fissured, while some forms may also include a submucous cleft into the hard palate, so that the bone defect is covered by a duplication of the mucous membrane (pellucid zone).
REFERENCES – EmbrYology and clinical features
Bernheim N, Georges M, Malevez C, De Mey A, Mansbach A. Embryology and epidemiology of cleft lip and palate. B-ENT. 2006; 2 Suppl 4:11-9.
Burdi AR. Cephhalometric growth analyses of the human upper face region during the last two trimesters of gestation. Am J Anat. 1969 May; 125(1): 113-22.
Gundlach KK, Pfeifer G. The arrangement of muscle fibres in cleft lips. J Maxillofac Surg. 1979 May; 7(2):109-16.
Marazita ML, Mooney MP. Current concepts in the embryology and genetics of cleft lip and cleft palate. Clin Plast Surg. 2004 Apr; 31(2):125-40.
Maue-Dickson W. The craniofacial complex in cleft lip and palate: an update review of anatomy and function. Cleft Palate J. 1979 Jul; 16(3):291-317.
3. CRANIOFACIAL GROWTH IN CLEFT LIP AND PALATE
(M.C. Meazzini)
Patients affected by CLP present an impaired craniofacial growth with a specific growth inhibition in the three planes of space.
GROWTH OF THE MAXILLA
Over 200 cephalometric studies have been conducted on patients with cleft lip and cleft palate, and all have shown significant differences in maxillary growth compared to normal subjects. Anomalies have been reported in shape, position and in size in the sagittal, transverse and vertical planes.
As regards to the shape, Laspos et al. (1997) describes the typical asymmetry of the naso-maxillary complex, which can increase with growth, reaching maximum levels after puberty.
Most authors have shown a reduction in the sagittal length of the maxilla, a more retruded position of the maxilla on the sagittal plane, together with the posterior rotation of the palatal plane (Ozturk and Cura, 1996, Capelozza Filho et al; 1996; Semb and Shaw, 1990)
Vertically, various authors have pointed out the reduction of the posterior maxillary height together with a decreased superior anterior facial height (Ozturk and Cura, 1996; Wade, 1997).
Since the 70’s in almost all studies a reduction in the transverse diameters of the naso-maxillary complex following the surgical correction of the cleft has been reported (Dahl, 1970).
The palates of affected patients are much narrower, more so anteriorly than posteriorly with a short palatal vault distal to the canines: the palatal vault is asymmetrical (Smahel et al, 2004).
To better understand the pathogenesis of such a frequent alteration of growth it is often useful to evaluate non operated individuals.
GROWTH IN UNTREATED SUBJECTS
All skeletal alterations of the upper jaw that have been discussed to this point refer to patients who have undergone surgical treatment. The possibility to find and collect samples of patients at the end of growth which have never been operated, delivers the unique opportunity to study the growth of cleft lip and palate patients without the interference of surgery. As often occurs in the medical literature, there is a large inconsistency of results, with sometimes controversial conclusions. Many studies have concluded that the sagittal growth of the midface of a patient with CLP is not congenitally inhibited. If not operated the jaw would reach the normal relations with the remaining facial formations (Ortiz-Monasterio et al, 1959; Hagerty and Hill, 1963; Boo-chai, 1971). Therefore, the presence of the cleft is not, according to these authors, a factor inhibiting growth processes. In the absence of surgery, the jaw would potentially be able to reach an almost normal antero-posterior dimension (Capelozza Filho et al, 1996). The same results were obtained by Shetye and Evans (2006), showing a remarkable protrusion of point A, compared to the control sample together with mandibular retrusion and a much wider cranial base angle.
On the other hand, Isiekwe and Sowemimo (1984), Bishara et al. (1986) and Yoshida et al. (1992), have reported in untreated adults with complete unilateral cleft lip and palate, that maxillary growth is indeed inhibited in an anterio-posterior direction and, while there is a marked dento-alveolar protrusion, the skeletal base is actually retruded.
As a matter of fact, many of the studies that support a normal growth potential, use cephalometric point A as a reference (which is a skeletal, but also an eminently dento-alveolar point) instead of the anterior nasal spine, as they deem ANS a cephalometric point which is insufficiently reliable (Shetye and Evans, 2006).
Therefore, the question of growth in untreated subjects remains open, but it is clear that the intrinsic factor is still only partially responsible for the growth results in patients which have undergone surgery.
FACTORS CONTRIBUTING TO MAXILLARY HYPOPLASIA IN CLP
With regards to the maxillary hypoplasia and the deformities of the midface in a patient with cleft lip and palate, 3 primary factors are involved: intrinsic factors, the result of a congenital dysmorphogenesis, functional adaptive factors, due to an aberrant post-natal growth and iatrogenic factors, induced by post-surgical scarring.
INTRINSIC FACTORS
Among the factors that contribute to the diminished growth of the maxilla, an intrinsic defect may not be ruled out. This might be the result of a deficiency of mesenchyme and migrating neural crest cells in the midfacial area during the embryonic stages of formation of the facial processes (Horswell and Gallup, 1992). This is confirmed by some of the studies on non operated patients (Isiekwe and Sowemimo, 1984, Bishara et al; 1986 and Yoshida, 1992). In a study conducted at San Paolo Hospital (Milano) a high correlation between congenitally missing laterals (neural crest migration defect), abnormal skull base and maxillary hypoplasia was noticed (Meazzini et al, 2008), also pointing out the importance of intrinsic factors in maxillary hypoplasia in CLP.
FUNCTIONAL FACTORS
According to Smahel et al. (2004), the size reduction in the transverse and in the vertical planes of the palate leads to a reduction of the space for the tongue, which, coupled with the high incidence of oral breathing in children with CLP, results in a lower and more anterior position of the tongue, thus removing the tongue support to the upper arch: This, in turn worsens the contraction of the transverse and sagittal diameters of the jaw.
As in the literature concerning children without cleft, extremely rigorous quantitative assessment of functional factors is very difficult to achieve and, therefore, no definitive conclusion over the actual influence of functional factors on maxillary growth of CLP patients has ever been drawn.
IATROGENIC FACTORS
Graber, in a pioneer study in 1949, concluded that surgery severely inhibits the growth of the midface and still at present all the authors are unanimous in considering surgical procedure as the most significant variable that affects craniofacial growth (Ross, 1987; Shaw et al, 1992; Semb and Shaw, 1996).
The tension exerted by post-operative scar tissue retraction would therefore, be the origin of diminished growth (Ozturk and Cura, 1996; Smahel and Mullerova 1996; Capelozza Filho et al., 1996).
What still remains controversial is whether the disturbances in the development of the midface are more related to the surgery of the lip or to palatal surgery or to the types of surgery or to their timing or the different techniques with which they are performed.
In the evaluation of the respective responsibility of the different types of surgery, the main problem is the fact that, in developed countries, at least cheiloplasty, but also surgery of the palate are usually carried out very early, well before the end of the growth of the facial complex, and consequently the effects observed may be attributed to both surgeries, without being able to separate them.
In developing countries, for reasons such as lack of resources, cultural perception or socio-economic situation, it is possible to find patients who have reached adulthood that have undergone only lip surgery. It is basically impossible to find groups of subjects operated exclusively on the palate, which would allow us to test the isolated effects of palatal surgery on growth.
EFFECTS OF LIP REPAIR
The repair of the lip, according to many authors is the surgical maneuver most responsible for maxillary hypoplasia in CLP (Hagarty and Hill, 1963; Bardach and Eisbach, 1977; Eisbach and Bardach, 1978; Bardach and Mooney, 1984; Bardach and Kelly, 1988; Capelozza Filho et al, 1996; Kapucu et al, 1996).
There are numerous authors who have examined the short-term effects of lip repair on dento-facial morphology in patients with UCLP (Mazaheri et al, 1993; Honda et al, 1995; Huang et al, 2002). Not as many studies have followed up the long-term effect of cheiloplasty on growth.
Capellozza Filho et al., in a study comparing unilateral non operated CLP patients to a sample of patients operated only at the lip to a sample of patients subjected to both lip and palatal surgery, concluded that lip surgery should be considered as the most important factor inhibiting the development of the maxilla, both at a basal and at a dento-alveolar level (Capellozza Filho et al, 1996), as they found no statistically significant difference between the two operated groups (lip surgery only or lip and palate surgery). Both groups significantly differed from the non operated sample especially in terms of reduction of the overjet and incisal retroclination.
Similarly, Kapucu compared UCLP patients subjected to lip surgery only, to a sample subjected to lip and palate surgery and comparing them with a group of normal subjects (Kapucu et al, 1996). The conclusions of this study were similar to those of the study mentioned above, namely that the cheiloplasty is the most important influencing factor on maxillary retrusion.
Conversely, in a recent study, Liao and Mars (2005) concluded that lip repair primarily induces an inclination of the alveolar process of the pre-maxilla, accompanied by a controlled uprighting of the upper incisors, with a decreased overjet. Therefore, the effect of cheiloplasty doesn’t seem to be exerted at the basal bone level, as the length and the anterior and posterior vertical height of the maxillary bone are the same in both groups. According to these authors, the earlier the repair of the lip, the greater the remodeling effect.
Even though results on the effect of cheiloplasty are contrasting, the debate is eminently an academic one, as it would be impossible and unethical not to perform a cheiloplasty till adolescence.
We shall therefore concentrate on the effect of palatoplasty.
EFFECTS OF THE PALATE REPAIR
Palate repair has considerable functional implications in CLP patients, especially in regards to the quality of speech, but also on secondary hearing impairment. It is therefore essential to close the palate early. However, this clashes with the numerous studies that demonstrated the negative influence on the growth of the maxilla of palate surgery (Schweckendiek, 1958 and 1978, Hotz and Gnionski 1976; Friede et al, 1980). The idea that the closure of the palate could be an important factor inhibiting the growth of the upper jaw arose from Hefert’s early clinical and experimental studies (Hefert, 1958). Hefert was the first author to suggest that the very nature of palatal surgery, which needs the lifting of muco-periosteal flaps, might damage the growth centers located in the hard palate. According to Ross (1970), the post-operative palatal scar inhibits subsequent growth of the maxilla.
Longitudinal studies have demonstrated a progressive deterioration in maxillary growth, especially during the adolescent growth spurt (Ross, 1987; Enemark et al, 1990; Semb, 1991; Smahel et al, 1992; Meazzini et al, 2008a).
Few studies have evaluated the long-term effect of palatal repair in order to compare it to the effect of lip repair. The main problem, as already mentioned in the chapter on cheiloplasty, is the difficulty in separating the effects of the two surgeries. The isolated effect of palate repair on craniofacial growth of patients with complete CLP remains essentially impossible to clinically assess, for the obvious lack of availability of a sample subjected only to palate repair and not to lip repair.
Liao and Mars (2005) have compared two groups of UCLP patients, where one group was subjected exclusively to a cheiloplasty, while the second group to a cheiloplasty together with the hard palate closure at the average age of nine years. The authors suggest that palate repair has an effect on the position of the maxillary skeletal base, which is more retruded in the sample subjected to palate repair. These results are in contrast with those reported by Kapuku and by Da Silva Filho, but it is almost impossible to understand the reason for this discrepancy, given the high number of variables in each study, not allowing a proper comparison.
The timing of palatoplasty is certainly one of the most controversial issues in the literature. Regarding the timing of palatal closure there are two different schools of thought. The first suggests to close the hard palate and the soft palate in two surgical steps, in order to delay, as much as possible, the closure of the hard palate. The rationale for this approach is based on the hypothesis that by delaying the closure of the hard palate there it’s disrupting effect on maxillary growth would be reduced (Schweckendiek, 1958 and 1978: Hotz and Gnionski, 1976; Friede et al, 1980). The second protocol prefers earlier hard palate closure, not long after lip repair, with the objective to minimize speech problems which arise by closing the hard palate too late (Noordhooff et al, 1983; Rohrich et al, 1996).
Ross (1987) in a fundamental study on the factors influencing maxillary growth in CLP, concluded that palatoplasty certainly inhibits maxillary growth and ideally should be performed after adolescence. But, of course, this is not possible, as it would be detrimental for speech. Interestingly, this author did not find much difference in facial growth with different timing of hard palate closure (10, 24 months, 5 or 9 years of age), concluding that there were too many other uncontrolled variables for an unbiased assessment on the timing of palatoplasty. Gaggl (2003) compared a sample where hard palate closure was executed at one year of age to one where the palate had been repaired at six years of age. This author did not find any difference in maxillary growth. The same conclusion was drawn by Rohrich (1996) in a study comparing maxillary growth in a sample where hard palate closure was performed at 3 months and at 4 years. This author therefore suggests that there is no indication for a delayed palatal closure, which inevitably induces speech problems. Although there is a cephalometrical flaw in the study, as unilateral and bilateral cleft lip and palate patients were put together in the same sample, the conclusion regarding speech is extremely important (Rohrich, 1996).
In contrast, as mentioned above, many authors favor later palatoplasty. Lilja et al. (2006) have shown that patients who have been subjected to late hard palate repair (9 years of age) have a better development of the midface compared to those where the hard palate was closed early (three months of age) with a vomer flap. Friede and Enemark (2001) suggested that the same results may be achieved without waiting till 9 years of age, but by performing the palatoplasty at 5 years.
So, which is the best timing for palate repair? When is early enough for good speech? When is late enough for acceptable growth?
A recent study, conducted with extreme scientific rigor, maybe helps us understand a little more (Liao at al, 2010). The authors have compared at 20 years of age patients whose palate had been repaired at 1 year and at 5 years. They concluded that maxillary growth was inhibited by an earlier repair, beause the patients who had undergone palate repair at 1 year, had an extra 20% need for orthognathic surgery. These authors, though, emphasize the important fact that, when assessing a protocol, speech is more important than growth. Therefore, as it is generally accepted that speech is damaged by a palate repair performed after 4 years of age (Noordhooff et al, 1983; Rohrich et al, 1996; Lohmander, 1998), these authors suggest not to wait after this age.
Many questions remain unanswered. Does the palate repair technique (vomer flap or mucoperiosteal palatal flap) influence differently growth? Does early soft palate closure truly reduce the impact of subsequent hard palate closure?
All of these factors are fundamental in the choice of a protocol, but unfortunately the inherent biases present in all retrospective studies, have not allowed for an answer at this time.
Currently 90% of the 201 European CLP centers close the hard palate before 3 years of age. Many North American centers have moved the time to 18 months, in order to minimize speech disturbances.
At the Regional center for CLP in San Paolo Hospital (Milano), Prof. Brusati until 2006 had selected a two phase protocol, with lip and soft palate closure at 6 months and hard palate and gingivoalveoloplasty (GAP) at 18-24 months (Chapter 4). Speech results of this protocol are good, but it is difficult to assess the effect of hard palate closure, as it is performed together with GAP, which seems to increase the need for osteotomy of about 15% (Meazzini, 2008c).
Since 2006, in patients with a palatal cleft narrower than 1 cm, Prof Brusati has introduced an “all in one protocol”, with cheilorhinoplasty and soft and hard palate closure (and simoultaneous GAP in 36% of cases) at 6 months of age.
This modification to the previous protocol, therefore, allows to solve the “problem cleft” in only one surgery in about 15% of all the complete UCLP at 6 months. 25% of complete UCLP patients will need a small bone graft at 6 years of age. Which means that 40% of the complete UCLP patients might need even less speech therapy than the remaining 60% of the complete unilateral CLP patients which will undergo the “classical” two stages protocol.
It is highly probable that these patients might have a higher need for final osteotomies, but this likelihood seems less important, when considering the fact that during their whole infancy they will only be subjected to either only one, or at the most, two surgeries with very little speech therapy.
An individualized protocol, although probably the best choice for CLP patients, who have an inherent variability in severity (Berkowitz, 2005), will make future comparisons between protocols even more difficult.
ALVEOLAR BONE GRAFTING
Secondary alveolar bone grafting, usually carried out before the eruption of the prominent canines, gives good stability to the maxillary stance, and allows to the eruption of the permanent canine in the cleft. According to Semb (1990) this procedure influences only minimally the growth of the maxilla on all planes the space. On the other hand, other studies have shown a reduction of the vertical growth of the maxilla after secondary bone grafting (Ross, 1987). In the past primary bone grafting has been adopted, simultaneously with the cheiloplasty, but all authors agree that this procedure severely inhibits maxillary growth (Suzuki, 1996; Ross, 1987; Molsted, 1992; Brattstrom, 1989).
PRIMARY PERIOSTEOPLASTY OR GINGIVOALVEOLOPLASTY
Primary periosteoplasty has been considered by many authors a damaging procedure for subsequent maxllary growth (Henkel and Gundlach, 1997; Berkowitz, 2004). On the contrary, other authors do not feel that primary periosteoplasty has any negative effect on maxillary growth (Lee, 2004). It must be noted that these authors have not examined patients at the completion of growth and, more importantly, in their sample there was an important selection bias, which deeply influences the results (primary gingivo-periosteoplasty was only performed in clefts which were not wider than 1 cm).
Periosteoplasty may be performed later, as an early secondary procedure, at 18-24 months. Two studies were conducted at the Regional Centre for CLP at San Paolo hospital (Milano) in order to investigate whether by delaying the periosteal alveolar closure the negative influence on maxillary growth would be reduced.
The first study compared a sample of UCLP patients at the end of growth subjected to cheiloplasty and soft palate closure at six months and to gingivoalveoloplasty (GAP) and hard palate closure at 18-24 months, with a sample operated with the same protocol by the same surgeon, with the exception that no GAP was performed during palatoplasty, but a later (9-11 years) secondary bone grafting was carried out. This first study has shown 13% more need for Le Fort I osteotomies in the patients who had undergone GAP (Meazzini, 2008 b).
Similar results were obtained from the comparison with a sample of unilateral cleft lip and palate patients operated with the Oslo protocol, which includes lip closure at 3 months of age, hard and soft palate repair at 18 months of age and bone graft between 8 and 11 years of age (Meazzini, 2008a).
CONCLUSIONS ON CRANIOFACIAL GROWTH IN CLEFT LIP AND PALATE PATIENTS
Although it seems clear, on one hand, that surgery might be the principal cause of growth disturbances in the maxilla of cleft lip and palate patients, it also appears obvious that it is still impossible to indicate one surgical protocol or surgical procedure as the main responsible for growth disturbances.
Within all these questions, the only certain answer is that the experience and ability of the surgeon, are by far the most important factors to allow for a better growth of these little patients (Shaw, 1992).
REFERENCES – GROWTH
Bardach J., Eisbach K.J. The influence of primary unilateral cleft lip repair on facila growth. Cleft Palate J. 1977 Jan; 14(1): 88-97.
Bardach J., Kelly K.M. The influence of lip repair with and without soft-tissue undermining on facial growth in beagles. Plast Reconstr Surg. 1988 Nov; 82(5): 747-59.
Berkowitz S., Mejia M., Bystrik A. A comparison of the effects of Latham-Millard procedure with those of a conservative treatment approach for dental occlusion and facial aesthetics in unilateral and bilateral complete cleft lip and palate: part I Dental occlusion. Plast Reconstr Surg. 2004; 113(1): 1-18.
Bishara S.E., Jakobsen J.R., Krause J.C., Sosa-Martinez R. Cephalometric comparisons of individuals from India and Mexico with unoperated cleft lip and palate. Cleft Palate J. 1986 Apr; 23(2): 116-25.
Boo-Chai K. The unoperated adult bilateral cleft of the lip and palate. Br J Plast Surg. 1971 Jul; 24(3): 250-7.
Brattstrom V., Mc William J. The influence of bone grafting on dental abnormalities and alveolar bone heigth in patients with unilateral cleft lip and palate. Eur J Orthod. II 1989; 351-58.
Capelozza Filho L., Normando A.D., da Silva Filho O.G. Isolated influences of lip and palate surgery on facial growth: comparison of operated and unoperated male adults with UCLP. Cleft Palate Craniofac J. 1996 Jan; 33(1): 51-56.
Dahl E. Craniofacial morphology in congenital clefts of the lip and palate. An X-Ray cephalometric study oh young adult males. Acta Odontol. Scand. 1970; 28: 57.
Enemark H., Bolund S., Jørgensen I. Evaluation of unilateral cleft lip and palate treatment: long term results. Cleft Palate J. 1990 Oct; 27(4): 354-61.
Friede H., Enemark H. Long-term evidence for favorable midfacial growth after delayed hard palate repair in UCLP patients. Cleft Palate Craniofac J. 2001 Jul; 38(4): 323-9.
Friede H., Lilja J., Johanson B. Cleft lip and palate treatment with delayed closure of the hard palate: a preliminary report. Scand J Plast Reconstr Surg. 1980; 14: 49-53.
Gaggl A., Schultes G., Feichtinger M., Santler G., Mossböck R., Kärcher H. Differences in cephalometric and occlusal outcome of cleft palate patients regarding different surgical techniques. J Craniomaxillofac Surg. 2003 Feb; 31(1): 20-6.
Graber T.M. Craniofacial morphology in cleft palate and cleft lip deformities. Surg Gynecol Obstet. 1949 Mar; 88(3): 359-69.
Hagerty R.F., Hill M.J. Facial growth and dentition in the unoperated cleft palate. K. Dent. Res. 1963; 42: 412.
Henkel K.O., Gundlach K.K. Analysis of primary gingivoperiosteoplasty in alveolar cleft repair. Part I: facial growth. J Cranio Maxillofac Surg. 1997; 25(5): 266-9.
Honda Y., Suzuki A., Ohishi M., Tashiro H. Longitudinal study on the changes of maxillary arch dimensions in Japanese children with cleft lip and/or palate: infancy to 4 years of age. Cleft Palate Craniofac J. 1995 Mar; 32(2): 149-55.
Horswell B.B., Gallup B.V. Cranial base morphology in cleft lip and palate: a cephalometric study from 7 to 18 years of age. J Oral Maxillofac Surg. 1992 Jul; 50(7): 681-5 (discussion 686).
Hotz M., Gnoinski W. Comprehensive care of cleft lip and palate children at Zürich university: a preliminary report. Am J Orthod. 1976 Nov; 70(5): 481-504.
Huang C.S., Wang W.I., Liou E.J., Chen Y.R., Chen P.K., Noordhoff M.S. Effects of cheiloplasty on maxillary dental arch development in infants with unilateral complete cleft lip and palate. Cleft Palate Craniofac J. 2002 Sep; 39(5): 513-6.
Isiekwe M.C., Sowemimo G.O. Cephalometric findings in a normal Nigerian population sample and adult Nigerians with unrepaired clefts. Cleft Palate J. 1984 Oct; 21(4): 323-8.
Johnson G.P. Craniofacial analysis of patients with complete cleft of the lip and palate. Cleft Palate J. 1980 Jan; 17(1): 17-23.
Kapucu M.R., Gürsu K.G., Enacar A., Aras S. The effect of cleft lip repair on maxillary morphology in patients with unilateral complete cleft lip and palate. Plast Reconstr Surg. 1996 Jun; 97(7): 1371-5.
Laspos C.L., Kyrkanides S., Tallents R.H., Moss M.E., Subtelny J.D. Mandibular and maxillary asimmetry in individuals with unilateral cleft lip and palate. Cleft Palate Craniofac J. 1997 May; 34(3): 232-239.
Lee CT, Grayson BH, Cutting CB, Brecht LE, Lin WY. Prepubertal midface growth in unilateral cleft lip and palate following alveolar molding and gingivoperiosteoplasty. Cleft Palate Craniofac J. 2004 Jul;41(4):375-80.
Liao Y.F., Mars M. Long-term effects of lip repair on dentofacial morphology in patients with unilateral cleft lip and palate. Cleft Palate Craniofac J. 2005 Sep; 42(5): 526-32.
Liao YF, Yang IY, Wang R, Yun C, Huang CS. Two-stage palate repair with delayed hard palate closure is related to favorable maxillary growth in unilateral cleft lip and palate. Plast Reconstr Surg. 2010 May;125(5):1503-10.
Lilja J, Mars M, Elander A, Enocson L, Hagberg C, Worrell E, Batra P, Friede H. Analysis of dental arch relationships in Swedish unilateral cleft lip and palate subjects: 20-year longitudinal consecutive series treated with delayed hard palate closure. Cleft Palate Craniofac J. 2006 Sep;43(5):606-11.
Lisson J.A., Hanke I., Trankmann J. Vertical changes in patients with complete unilateral and bilateral cleft lip, alveolus and palate. J Orofacial Orthop. 2004 May; 65(3): 246-58.
Lohmander-Agerskov A. Speech outcome after cleft palate surgery with the Göteborg regimen including delayed hard palate closure. Scand J Plast Reconstr Surg Hand Surg. 1998 Mar;32(1):63-80.
Mazaheri M., Athanasiou A.E., Long R.E. Jr., Kolokitha O.G. Evaluation of maxillary dental arch form in unilateral clefts of lip, alveolus, and palate from one month to four years. Cleft Palate Craniofac J. 1993 Jan; 30(1): 90-3.
Meazzini M.C, Giussani G., Morabito A., Semb G., Garattini G., Brusati R. A cephalometric intercenter comparison of patients with unilateral cleft lip and palate: analysis at 5 and 10 years of age and long term. Cleft Palate Craniofac J. 2008 Nov;45(6):654-60.
Meazzini M.C., Capasso E., Morabito A., Garattini G., Brusati R. Comparison of growth results in patients with unilateral cleft lip and palate after early secondary gingivoalveoloplasty and secondary bone grafting: 20 years follow up. Scand J Plast Reconstr Surg Hand Surg. 2008;42(6):290-5.
Meazzini M.C., Donati V, Garattini G., Brusati R. Maxillary growth impairment in cleft lip and palate patients: a simplified approach in the search for a cause. J Craniofac Surg. 2008 Sep;19(5):1302-7.
Meazzini M.C., Tortora C., Morabito A., Garattini G., Brusati R. Alveolar bone formation in patients with unilateral and bilateral cleft lip and palate after early secondary gingivoalveoloplasty: long-term results. Plast Reconstr Surg. 2007 Apr 15; 119(5): 1527-37.
Mølsted K., Asher-McDade C., Brattström V., Dahl E., Mars M., McWilliam J., Plint D.A., Prahl-Andersen B., Semb G., Shaw W.C., e col. A six-center international study of treatment outcome in patients with clefts of the lip and palate: Part 2. Craniofacial form and soft tissue profile. Cleft Palate Craniofac J. 1992 Sep; 29(5): 398-404.
Noordhoff M.S., Kuo J., Cheng W.S. Results of the Widmaier-Perko palatoplasty in clefts of secondary palate. Ann Acad Med Singapore. 1983 Apr; 12(2 Suppl): 359-62.
Ortiz-Monasterio F., Rebeil A.S., Valderrama M., Cruz R. Cephalometric measurements on adult patients with non-operated cleft palates. Plast Reconstr Surg Transplant Bull. 1959 Jul; 24(1): 53-61.
Oztürk Y., Cura N. Examination of craniofacial morphology in children with unilateral cleft lip and palate. Cleft Palate Craniofac J. 1996 Jan; 33(1): 32-6.
Rohrich R.J., Rowsell A.R., Johns D.F., Drury M.A., Grieg G., Watson D.J., Godfrey A.M., Poole M.D. Timing of hard palatal closure: a critical long term analysis. Plast Reconstr Surg 1996; 98: 236-46.
Ross R.B. The clinical implications of facial growth in cleft lip and palate. Cleft Palate J. 1970; 7: 37.
Ross R.B. Treatment variables affecting facial growth in complete unilateral cleft lip and palate. Cleft Palate J. 1987 Jan; 24(1): 5-77.
Schweckendiek W. Ergebnisse die lippen-kiefer-gaumans palatoperationem mit der primaren veloplastik. Fortsh Kieper U.G. 1958; 4: 167-179.
Schweckendiek W. Primary veloplasty: long term results without maxillary deformity, a twenty-five year report. Cleft Palate J. 1978; 5(3): 268-274.
Semb G. A study of facial growth in patients with bilateral cleft lip and palate treated by the Oslo CLP Team. Cleft Palate Craniofac J. 1991 Jan; 28(1): 22-39.
Semb G., Shaw W.C. Pharyngeal flap and facial growth. Cleft Palate J. 1990 Jul; 27(3): 217-24.
Semb G., Shaw WC. Facial growth in orofacial clefting disorders. In: Turvery VigKWL, Fonseca RJ ed, Facial cleft and Craniostenosis. Philadelphia: Saunders, 1996: 28-56.
Shaw W.C., Dahl E., Asher-McDade C., Brattstroem V., Mars M., McWilliams J., Molsted K., Plint D.A., Prhal-Andersen B., Roberts C., Semb G. A six-center international study of treatment outcome in patients with clefts of the lip and palate; Part 5. General discussion and conclusions. Cleft Palate Craniofacial J. 1992; 29: 413-418.
Shetye P.R., Evans CA. Midfacial morphology in adult unoperated complete unilateral cleft lip and palate patients. Angle Orthod. 2006 Sep; 76(5): 810-6.
Smahel S., Mullerova Z. Effects of primary periosteoplasty on facial growth in UCLP: 10 year follow-up. Cleft Palate J. 1988; 25: 356.
Smahel S., Mullerova Z. Facial growth and development in UCLP during the period of puberty: comparison of the development after periosteoplasty and after bone grafting. Cleft Palate Craniofac J. 1994 Mar; 31(2): 106-115.
Smahel Z., Machová P., Müllerová Z., Skvarilová B. Growth and development of the face in complete unilateral cleft lip and palate during prepubertal and pubertal periods. Acta Chir Plast. 1992; 34(3): 163-77.
Smahel Z., Trefny P., Formanek P., Mullerova Z., Peterka M. Three-dimensional morphology of the palate in subjects with unilateral complete cleft lip and palate at the stage of permanent dentition. Cleft Palate craniofac J. 2004 Jul; 41(4): 416-23.
Suzuki A., Goto K., Nakamura N., Honda Y., Ohishi M., Tashiro H., Fujino H. Cephalometric comparison of craniofacial morphology between primary bone grafted and nongrafted complete unilateral cleft lip and palate adults. Cleft Palate Craniofac J. 1996 Sep; 33(5): 429-435.
Wada T., Satoh K., Tachimura T., Tatsuta U. Comparison of nasopharyngeal growth between patients with clefts and noncleft controls. Cleft Palate Craniofac J. 1997 Sep; .34(5): 405-9.
Yoshida H., Nakamura A., Michi K., Wang G.M., Liu K., Qiu W.L. Cephalometric analysis of maxillofacial morphology in unoperated cleft palate patients. Cleft Palate Craniofac J. 1992 Sep; 29(5): 419-24.
4. Surgical treatment of cleft lip and palate patients
(R. Brusati)
Cleft lip and palate is the most frequent congenital deformity of the face (1/700 births) and has been a surgical problem since ancient times. A Chinese document dated 390 BC, reports on a dignitary of the Emperor who had been operated for a deformity of the lip. Other historical documents describe similar surgical procedures during the Greek and Roman times.
In 1700 Paré, a French surgeon, presented a surgical technique which consisted of discarding the margins of the cleft and then resuturing them with a complex 8-shaped ligature threaded through one or more angles inserted in the margins of the cleft. The key revolution of the treatment is due to the anatomical studies of Victor Veau, a French surgeon that in the 1930’s developed the basis for the rational treatment of the cleft lip and palate. The most important points of Veau’s philosophy are:
- There is no lack of tissue; tissues are retracted and dislocated, but present
- In order to repair the cleft lip and palate correctly it is essential to restore the anatomy of the dislocated structures
- An incorrect craniofacial growth and dysfunction are mainly a consequence of an incorrect anatomical reconstruction
Even though these principles are universally recognized as the base of cleft lip and palate repair, there are numerous different techniques and protocols used for the repair of cleft lip and palate (Eurocleft reported 194 different protocols in 201 European centers).
In this chapter I will describe my surgical experience that, based on more than 1000 of patients, has been continuously developing; I will describe just the more significant modifications. I have samples composed of groups of homogeneous patients that allowed me to conduct long-term studies on a variety of aspects (craniofacial growth, speech, nasolabial morphology, hearing, etc…). It is fundamental to emphatise the fact that the treatment of cleft lip and palate must be carried out by a team able to treat at least 30 new patients per year as stated by the Eurocleft (Shaw, 2001) and that this treatment must be carried out in centers where different specialties can work together alongside from the neonatologist and the surgeon, such as the orthodontist, the speech and language therapist and the ENT surgeon.
The Hospital I work in, the San Paolo Hospital in Milan, is the a regional centre for cleft lip and palate and treats over 80 new patients per year. We have a multidisciplinary team which consists of all the specialties mentioned above.
Evolution of a philosophy
My first approach to cleft lip and palate treatment, in the 1970s, was developed under the guidance of my tutors (Rusconi, Sanvenero, Rehrman, Perko), and followed the protocol more widely applied in Europe at that time, developed from the work of Schweckendiek (1955).
At birth or during the first few weeks of life a passive orthopedic plate was applied (Hotz, 1976) to guide the maxillary growth and narrow the width of the cleft prior to the surgical procedure (see chapter 5 presurgical orthopedics).
The surgical repair used to be carried out at six months of age for the closure of the soft palate and the lip at the same time. At around six years of age closure of the hard palate was performed and between nine and eleven years of age a bone graft in the alveolar cleft was performed. At 16-17 years of age a secondary correction of the nasal deformity, not previously corrected, was carried out. This philosophy was strictly followed without substantial variation untill 1987; in that year I obtained a fellowship in Nantes with Delaire.
I could then embrace a different philosophy close to Veau’s principles (Delaire, 1975) which consisted of the repair of the soft palate, lip and nose at 4-6 months of age in a single step procedure and then the repair of the hard palate and the alveolar cleft (gingivoalveoloplasty) between 18 and 24 months, thus avoiding a third surgery of bone graft and significantly reducing the nasal deformity. Therefore since 1988 I have followed this protocol (Brusati e Mannucci, 1992).
In 2004 the classic technique of palate repair was substantially modified with the introduction of an intra-velar palatoplasty according to Sommerlad (2003) performed with the use of a microscope.
In 2006, following the experience of DeMey (2006), I introduced the method ”all in one” (soft palate, hard palate with vomer flap, lip, rhinoseptoplasty) for the clefts not wider than 1 cm. Some of these could also undergo a simultaneous gingivoalveoloplasty.
With this new evolution the number of necessary surgical procedures for the correction of the deformity was significantly reduced, limiting the psychological costs (for the child and the family) together with the economical costs for the healthcare system.
Any potential negative effect on maxillary growth (in a small number of patients) can be corrected later after growth has ceased through the common orthognatic procedures.
Cheilorhinoplasty in unilateral cleft lip (in two stages)
First stage
At around 4-6 months, usually after the orthopedic treatment, the first stage of repair starts with the palatoplasty.
Once the soft palate is infiltrated with local anesthetic and adrenaline, the margins of the cleft are incised from the uvula backwards to the posterior third of the hard palate and, with a periosteal elevator, a oral mucoperiosteal flap is elevated from the horizontal process of the palatine bone with a particular care in remaining under the periosteum.
The periosteum is then lifted posteriorly to expose the palatine aponeurosis and the tendon of the tensor palati muscle.
Following this plane posteriorly the muco-glandular oral layer is separated from the muscular layer up to the base of the uvula.
The tendon of the tensor palati muscle is dissected medially to the pterygoid hook in order to easily separate the mucoperioseum of the nasal side from the medial aspect of the pterygoid process and then from the palatine bone.
Once the same procedures have been performed on the other side, then the nasal layer is sutured from the posterior third of the hard palate to the tip of the uvula without any tension.
At this point, with a microscope, the palatine musculature is dissected from the nasal floor and then rotated towards the midline with a posterior dislocation.
An accurate suture superimposing on the midline of the two muscular flaps completes the muscular reconstruction.
The mucoperiosteal flap can be freed in the retrotuberal region with curved scissors and then the oral layer can be sutured, most of the time without any need for release incision, untill the posterior third of the palate covering the nasal layer previously restored. At this point the cheilorinoplasty is performed.
Firstly the anatomical landmarks that will guide the reconstruction are identified: the peak of cupid’s bow of the healthy side, the centre line of the Cupid’s bow and the peak of Cupid’s bow on the cleft side on both the inner and the external stump.
The base of the columella and the base of the nasal ala on the affected side are drawn. The incisions are now drawn as described by Millard, the skin incision is carried out on both sides of the cleft and then the vestibular mucosa on the cleft side is incised before performing a wide subperiosteal dissection. At the base of the nasal ala, the transverse muscle and the external orbiculare muscle are identified.
Through a mucosal incision on the internal side, the anterior nasal spine area is widely exposed and then, staying on the midline, the nasal cartilagineal structures are dissected: the medial crus of the alar cartilages, the dome and the lateral crus on the cleft side.
A bilateral subpericondreal dissection of the nasal septum allows the luxation of its base from its attachment to the premaxilla and the vomer and its spontenous relocation on the midline.
The next step is the reconstruction of the vestibular mucosa, the anterior nasal floor and the muscular planes as suggested by Talmant (1993) and finally the closure of the skin with a particular care to the symmetry of the Cupid’s bow.
When an extra lengthening of the cleft side is needed, it can be obtained with a Z-plasty in the inferior part of the lip.
Thanks to the muscular reconstruction the nose usually shows a good symmetry and a few transfixing stitches of the cartilage may further improve the results.
After the operation the child does not need any specific restraint and normal feeding can be started after a few hours.
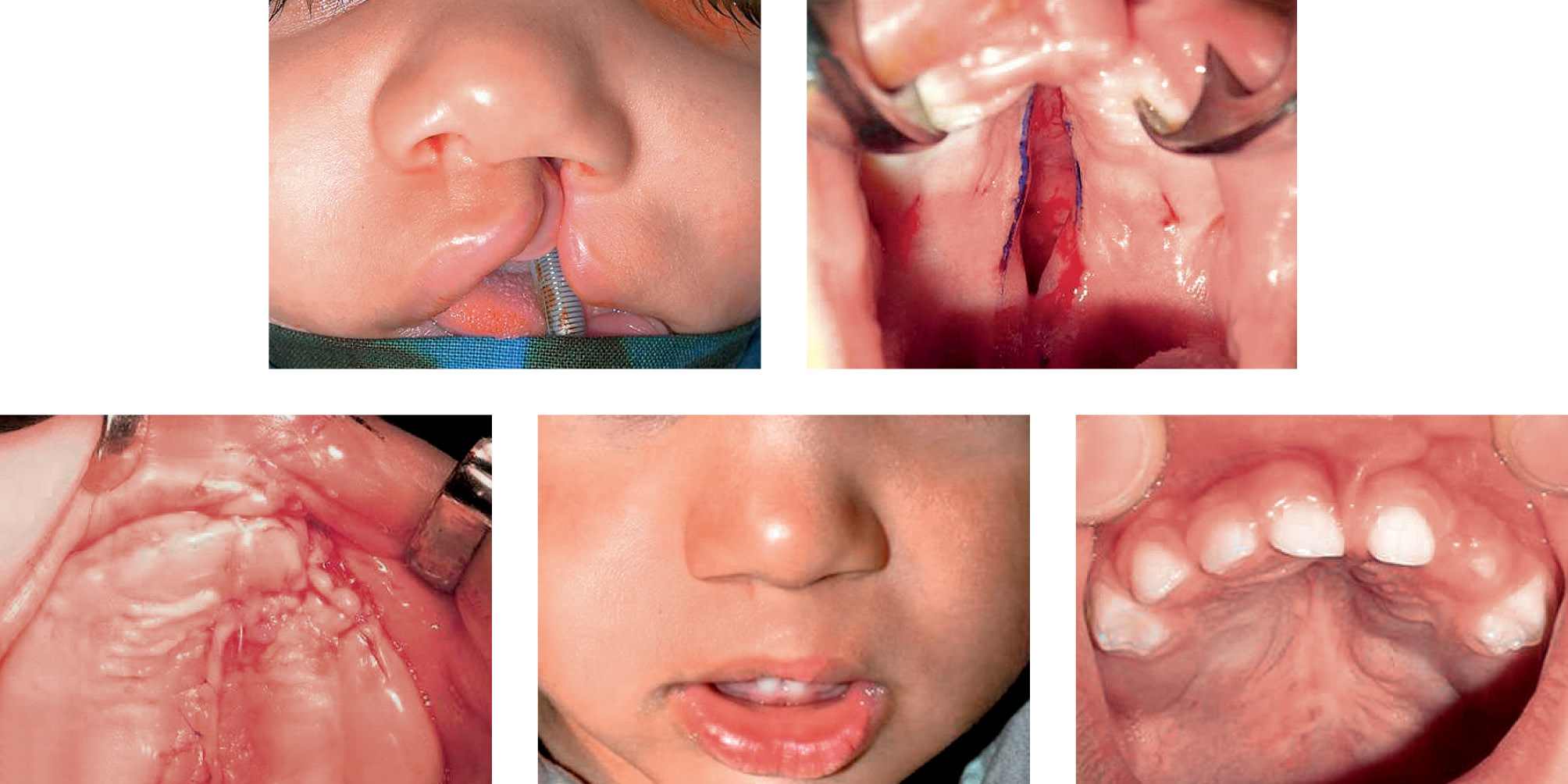
Case 1
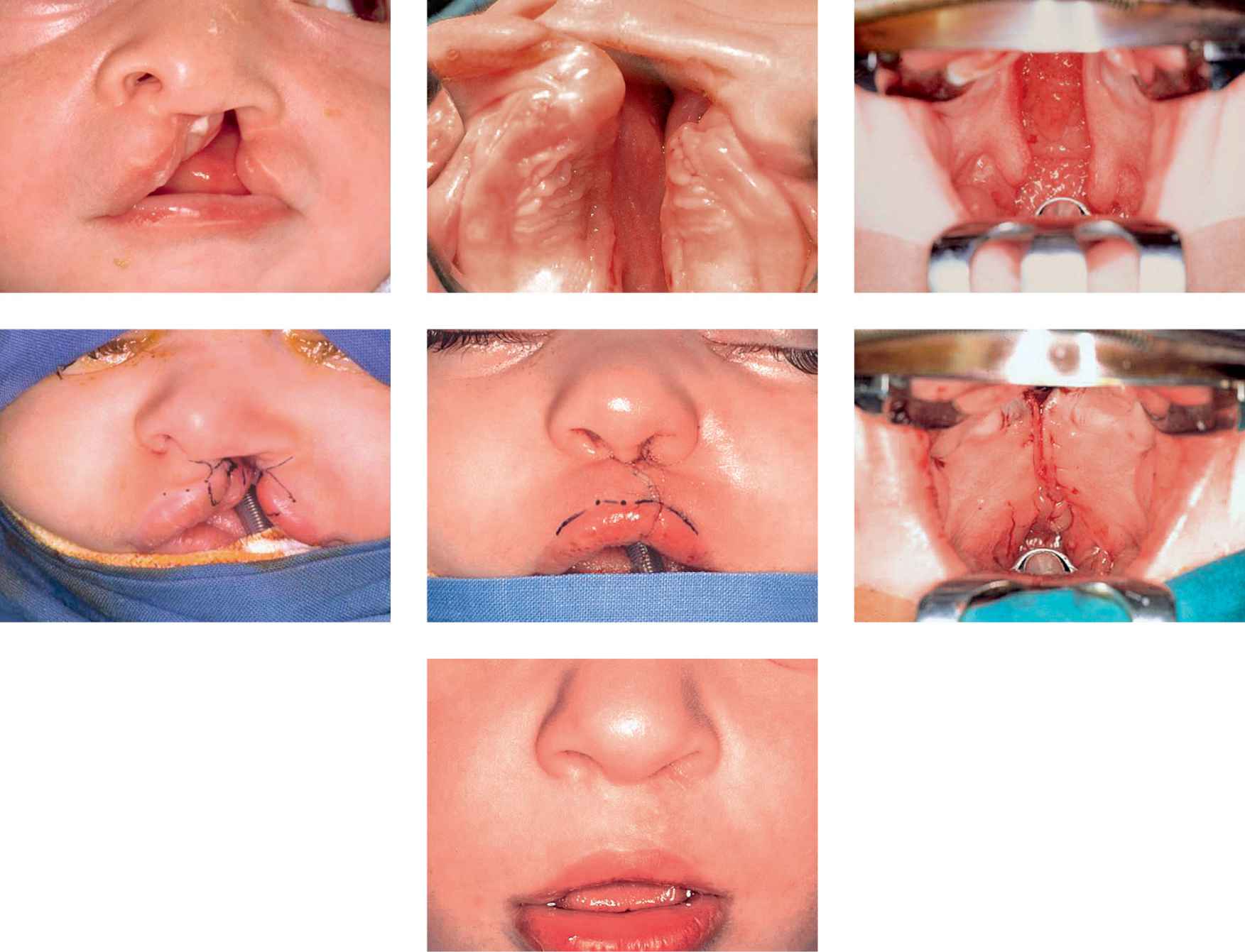
Case 1a-g • SLeft side unilateral cleft lip and palate: lip, soft and hard palate preoperative view, immediate postoperative view (first stage) and 4 years after surgery.
Second stage
At 18-24 months the modeling action of the reconstructed labial and palatal muscular cinguli the width of the residual cleft of the alveolus and the hard palate is usually significantly reduced to few millimeters and in some cases the cleft borders are into contact and the gingivoalveoloplasty and palatoplasty can be carried out without lateral release incisions (cases 2 and 3).
CASE 2
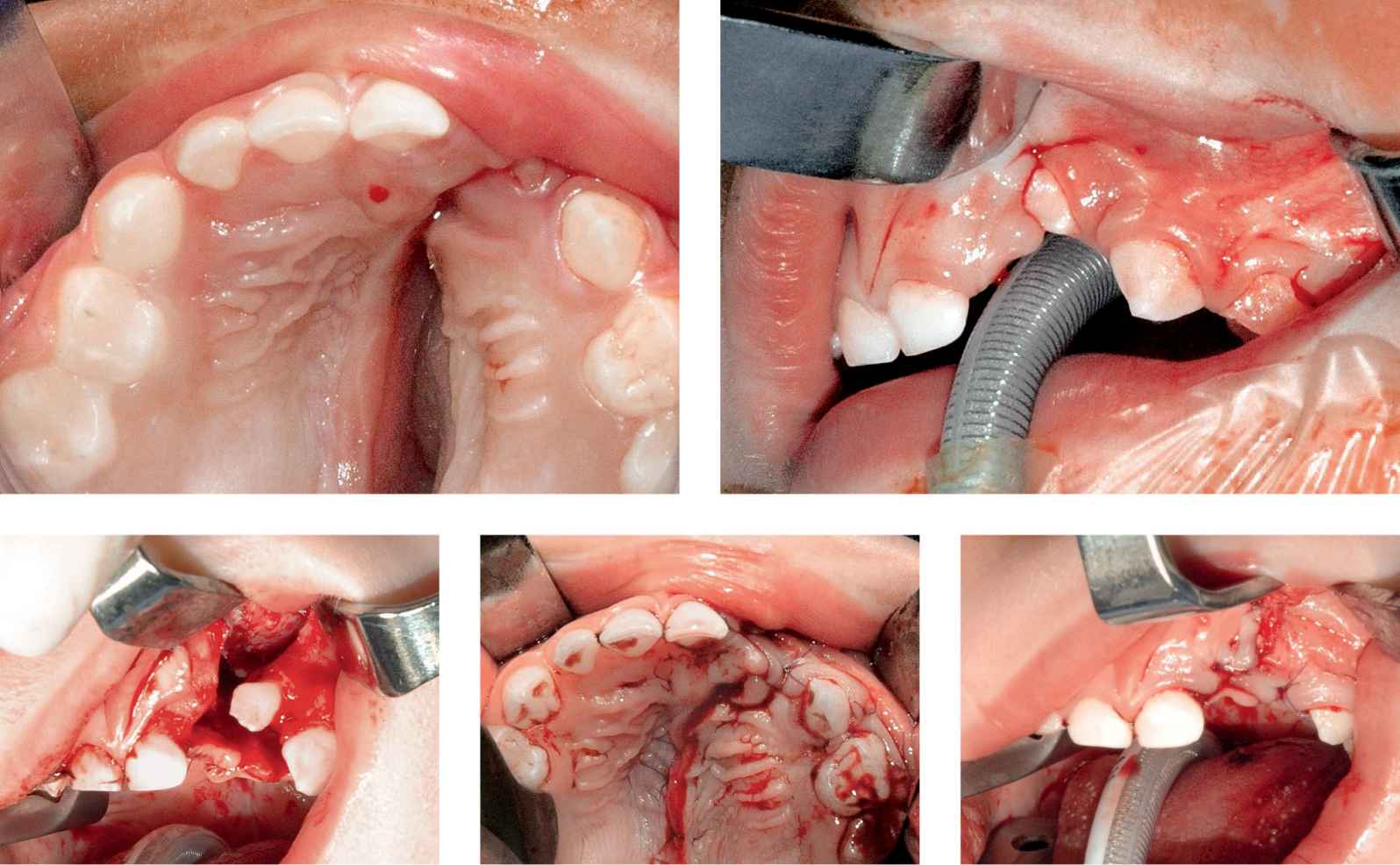
Case 2a-e • Left side unilateral cleft lip and palate: alveolar and hard palate appearance before, during and after hard palate repair and GAP (second stage).
CASE 3
Case 3a-d • Right side unilateral cleft lip and palate: alveolar and hard palate appearance showing excellent alveolo-maxillary ossification and normal upper maxillary growth in long-term follow-up after GAP.
We start with an intrasulcular incision on the small stump that goes from the canine to the second molar tooth and a vestibular mucoperiosteal flap is lifted after the incision is completed on the vestibular mucosa on the border of the alveolar cleft.
A vestibular flap is also lifted on the main stump with an intrasulcular incision at the level of the lateral (when present) or the central incisor that continues along the border of the alveolar cleft and meets the previously made incision to lift the small stump flap.
On the palatal side, with the incision along the margins of the cleft, it is possible to elevate an oral mucoperiosteal flap on both the main and the small stumps.
The nasal mucoperiosteum is dissected at the level of the palatine process and the nasal septum and then an accurate suture of the whole nasal layer is carried out.
Subsequently the oral palatal layer is sutured hermetically through U-stitches and finally the vestibular mucosa is sutured pulling together the two periosteal flaps in order to obtain the continuity of the adherent fibromucosa across the alveolar cleft.
In order to avoid any tension it is crucial to mobilize the distal flap through a horizontal incision of the periosteum; this is to enable repair of the palatal cleft and also to obtain an empty space of the alveolus completely lined by periosteum which has intrinsic osteoblastic activity and will avoid the need for secondary bone graft.
CHEILORHINOPALATOPLASTY IN ONE STAGE (ALL IN ONE)
When the palatal cleft width is less than 10 -12 mm, it is possible to repair the whole cleft in one single surgical stage and if the stumps at the alveolar level are in contact it is also possible to perform a primary gingivoalveoloplasty (case 4).
CASE 4
Case 4a-e • Left side unilateral cleft lip and palate: “all in one” procedure (cheilorhinoplasty, hard and soft palate repair, GAP); 2 years postoperative view.
We start, as in the two-stage procedure, by repairing the soft palate, but the incisions along the cleft margins are extended anteriorly to the alveolar process.
On the small stump the oral mucoperiosteum is elevated for few millimeters while, on the main stump, the mucoperiosteoum of the septum is horizontally folded underneath the palatal periosteum of the small stump; this enable the nasal plane to be completed along the whole length of the cleft from the soft palate to the alveolar process. Performing the cheilorynoplasty the suture of the nasal plane is extended anteriorly to the base of the nostril completing the closure of the nasal plane that will heal by secondary intention at the level of the hard palate and the alveolus. When the two stumps are in contact or very close to each other at the level of the alveolar process a simultaneous gingivoalveoloplasty can be performed through an incision of the alveolar mucoperiosteum on the edges of the alveolar processes and the suture of the vestibular and palatal flaps. When the gingivoalveoloplasty is not possible a secondary bone graft is usually performed at 6 years of age.
Cheilorhinoplasty in a bilateral cleft (in two stages)
Bilateral clefts present specific features that make their treatment extremely complex and mean the results are not as satisfactory as the treatment of the unilateral cleft lip and palate patients which are often barely visible once the treatment itself is completed.
The philosophy of treatment is the two stage procedure of the unilateral clefts but the preoperative orthopedic treatment aims not only to narrow the width of the cleft but also lengthen the columella, which is usually extremely short (chapter 5, presurgical orthopedics). However, frequently, it is necessary to operate on a bilateral cleft without any preoperative orthopedic treatment (cases 5, 6).
CASE 5
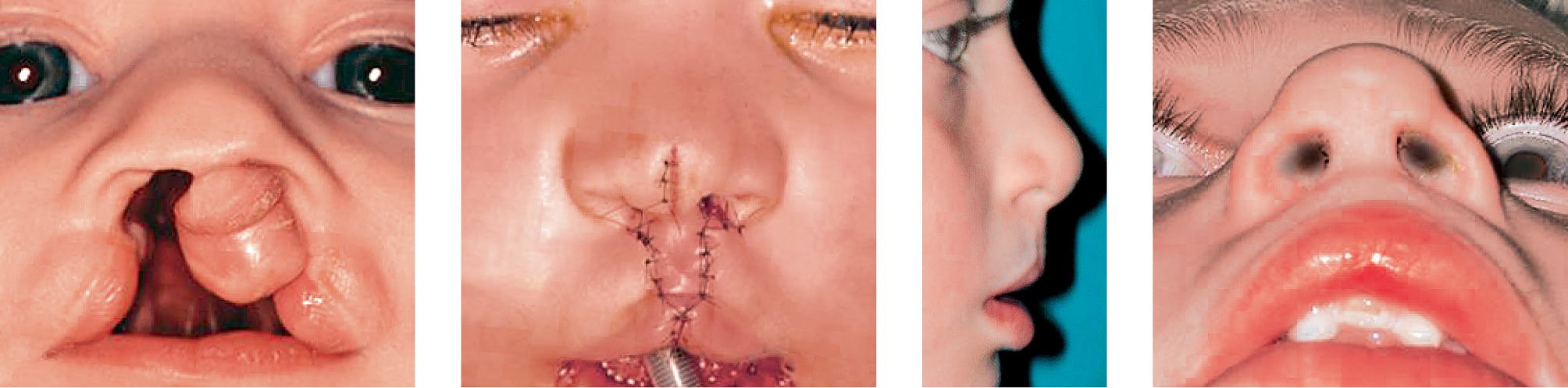
Case 5a-d • Bilateral cleft lip and palate: cheilorhinoplasty with primary lengthening of the columella with Mulliken’s technique: 5 years postoperative view.
CASE 6
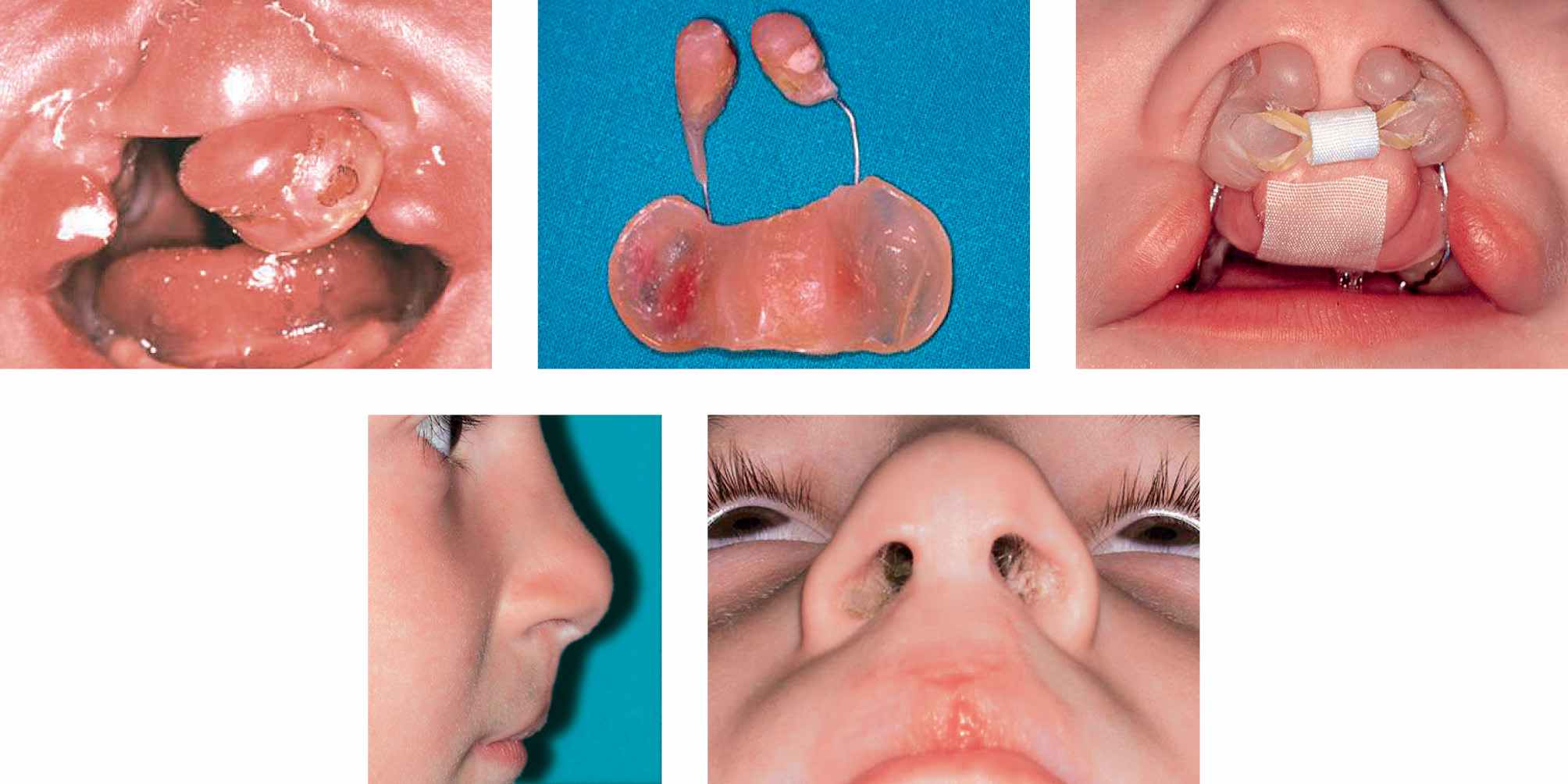
Case 6a-e • Bilateral cleft lip and palate: cheilorhinoplasty with primary lengthening of the columella with Cutting-Greyson technique: 8 years postoperative view.
First stage
In all patients the operation starts with the soft palate repair as previously described. Secondly the lip and nose are repaired.
In patients with extreme protrusion of the premaxilla it is necessary to carry out a lip adhesion through which, with a simple mucosal and cutaneous suture along the two thirds of the height of the lip the cleft is converted into an incomplete cleft, obtaining in a few months a significant retraction of the premaxilla and therefore a more adequate anatomical condition for the definitive lip repair.
The drawings derive from the Delaire technique (1991) for the lateral stumps and the Mulliken technique (1995) for the prolabium.
The transversus nasi and the external orbicularis muscles are identified and then the prolabium is elevated.
The Mulliken’s technique does not include the use of the orthopedic lengthening of the columella; this is obtained through endonasal marginal incisions with no need to go further to the anterior nasal spine, dissecting the skin from the alar cartilages and suturing the domes on the midline.
Over this cartilagineal support the cutaneous cover is brought together with the underlyng intercartilagineous adipose tissue and the endonasal marginal incisions are sutured with or without the resection of a small cutaneous portion.
The labial musculature is sutured in two layers: the deep layer is composed by the nasal transversi muscles sutured to the base of the nasal spine; the superficial layer is composed by the external orbicularis muscles sutured to each other and to the base of the nasal spine. Particular care is needed to tighten with the suture the base of the nostrils.
The suture of the skin restores the position of the labial philtri and the vermillion border, which is composed by the skin of two lateral stumps and the prolabial skin in its central triangular portion.
At the end of the procedure a useful silicon intranasal nasal moulding device is positioned.
When the patient has undergone the preoperative orthopedic treatment, the incisions are different at the level of the nasal access. In these cases the prolabial flap is lifted upward through a transfixed incision at the base of the cartilagineous septum so that the medial crus of the alar cartilages can be lifted with the prlabium.
Through a retrograde dissection between the skin and the alar cartilages (dome and lateral crus) the skin and the intradomes adipose tissue is freed and the two domes can be brought together and sutured with a U-stitch obtaining a significant lengthening of the columella.
The columella and prolabium are now repositioned trying to recreate a correct nasolabial angle with one stitch from the angle itself and the anterior nasal spine.
The labial muscles and skin are then reconstructed as previously described. The nasal moulding device is useful in these patients too.
Second stage
At 18-24 months a second procedure is carried out for the closure of the residual cleft of the hard palate and alveolomaxillary cleft.
The surgical technique is similar to the one described for the unilateral cleft but the preparation of the flaps of the premaxilla requires particular attention: in order to maintain an adequate blood supply to the premaxilla the flap dissection from the underlying bone on the vestibular side, at the level of the canine, has to be limited because it is provided purely by the vestibular side; on the palatal side it is necessary to design a wide fibromucosal flap with a large posterior base and lifted up to the incisive papilla in order to be able to suture it to the two lateral palatal flaps. To avoid oronasal communications it is extremely important to place a U-stitch between the three flaps corners in the midline. The vestibular suture is carried out as previously described.
Bone Graft
Even though our protocol consists of a gingivoalveoloplasty (GAP) that avoids the need for a bone graft, this can be necessary in patients treated under different protocols and in our “all in one” patients with no GAP. The aim is to restore the alveolar process and the base of the maxilla. This has a variety of indications: morphological (to provide more support for nasal ala), stability of the stumps, orthodontic (it gives the possibility of letting the teeth migrate into a correct alignment), implantophrostetic (it provides adequate bone support to insert an implant when the lateral incisor is missing).
In the literature there is agreement to perform the bone graft at 9-11 years of age when two thirds of the permanent canines roots have developed. Recently it is debated whether it is better to perform the operation before the eruption of the central incisor: it has been postulated that this would reduce the risk of periodontal desease and gum recession at the level of the central incisor on the cleft side.
From a technical point of view (Case 7) the flap design and suture are exactly the same as the GAP but with even more care and attention to obtain an hermetic closure of the nasal, palatal and vestibular planes since a small dehiscence can lead to the failure of the graft secondary to infection.
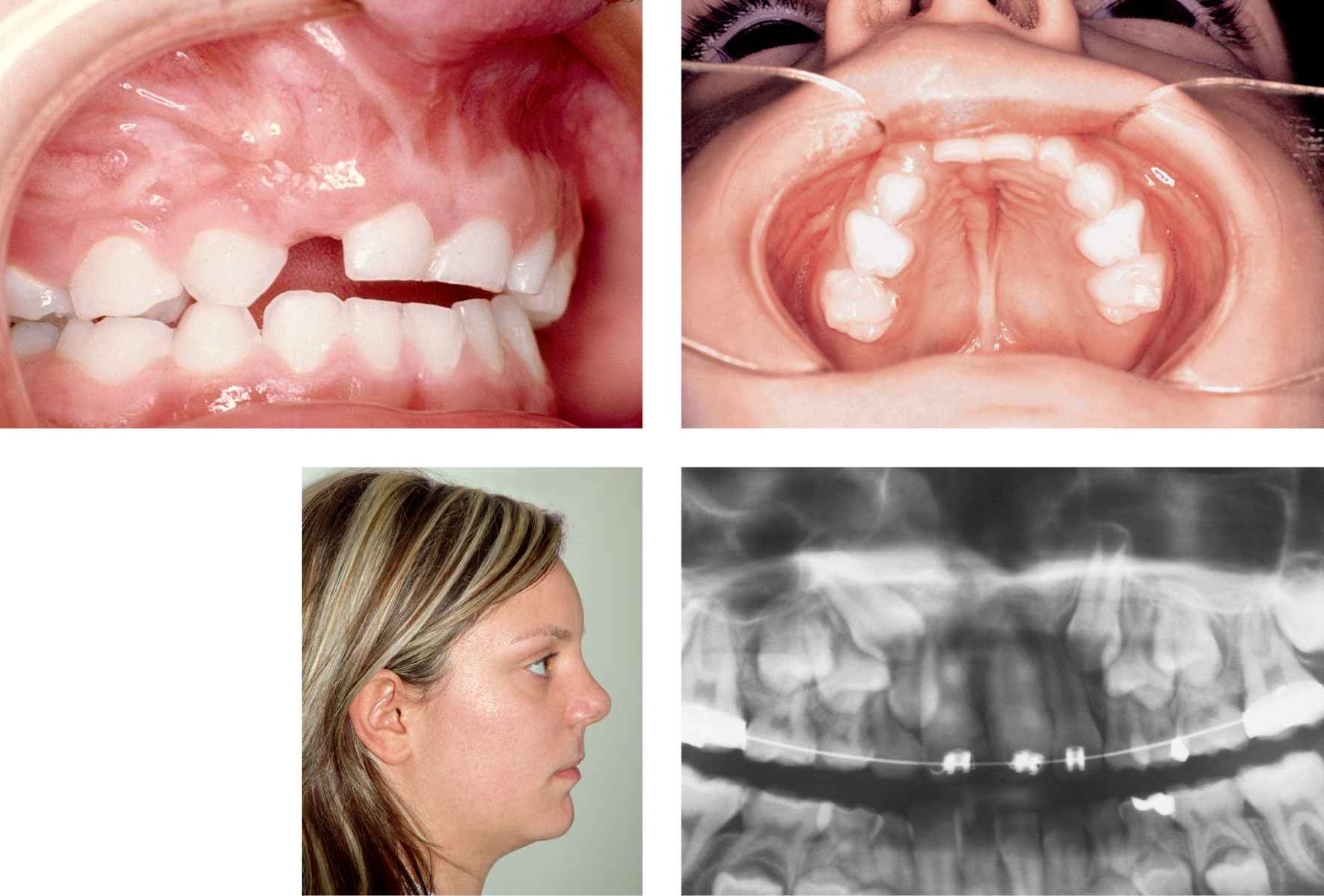
CASE 7
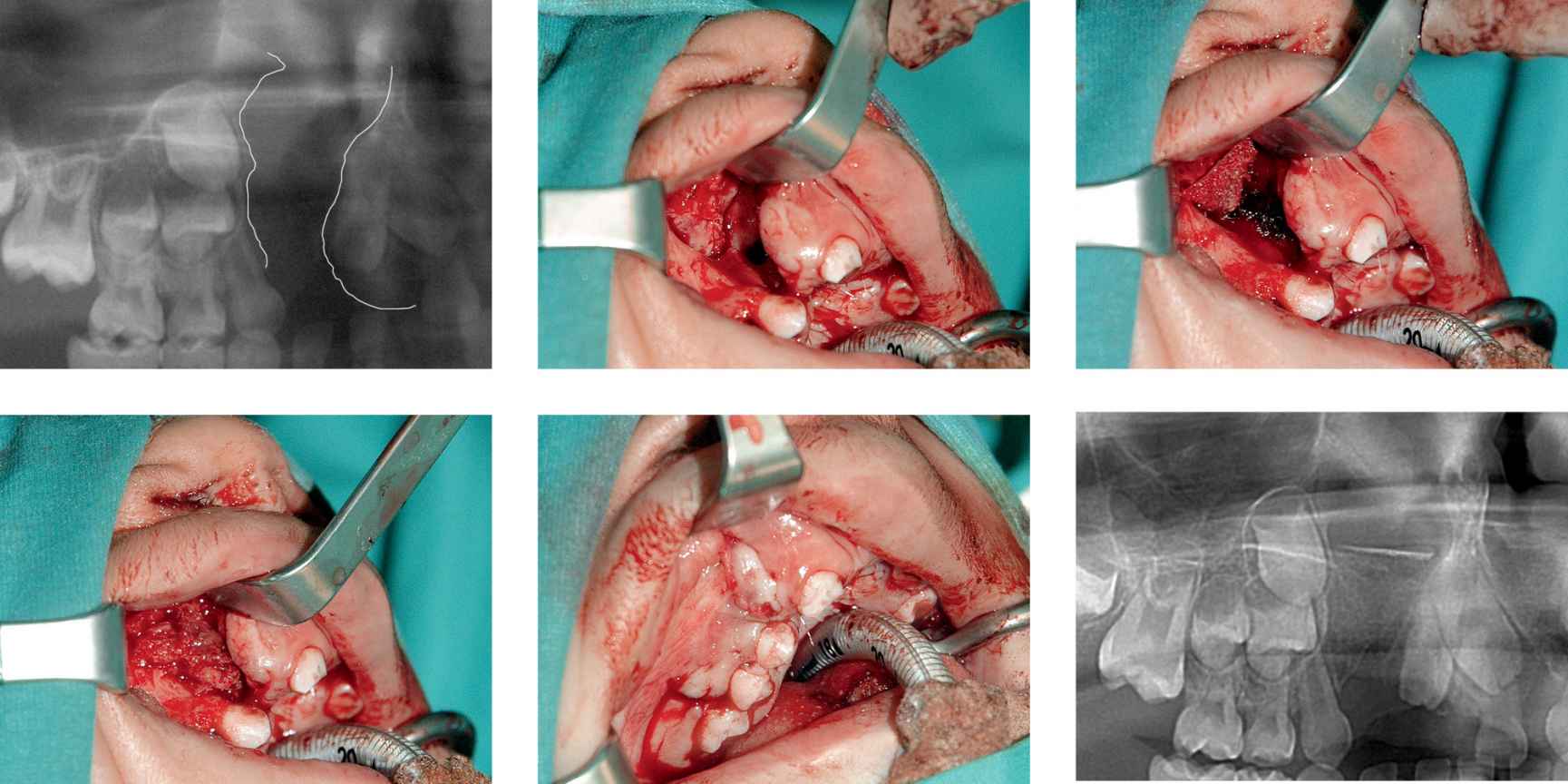
Case 7a-f • Right side alveolo-maxillary cleft: iliac crest cortico-spongious bone graft, intraoperative view.
In the wider and bilateral cleft the bone graft is usually harvested from the iliac crest with a cortical slat to reconstruct the nasal floor and a generous amount of spongious bone to reconstruct the alveolar process. For narrower clefts it can be sufficient to harvest bone from the mandibular symphysis with care not to damage the teeth apexes, in particular the unerupted canines.
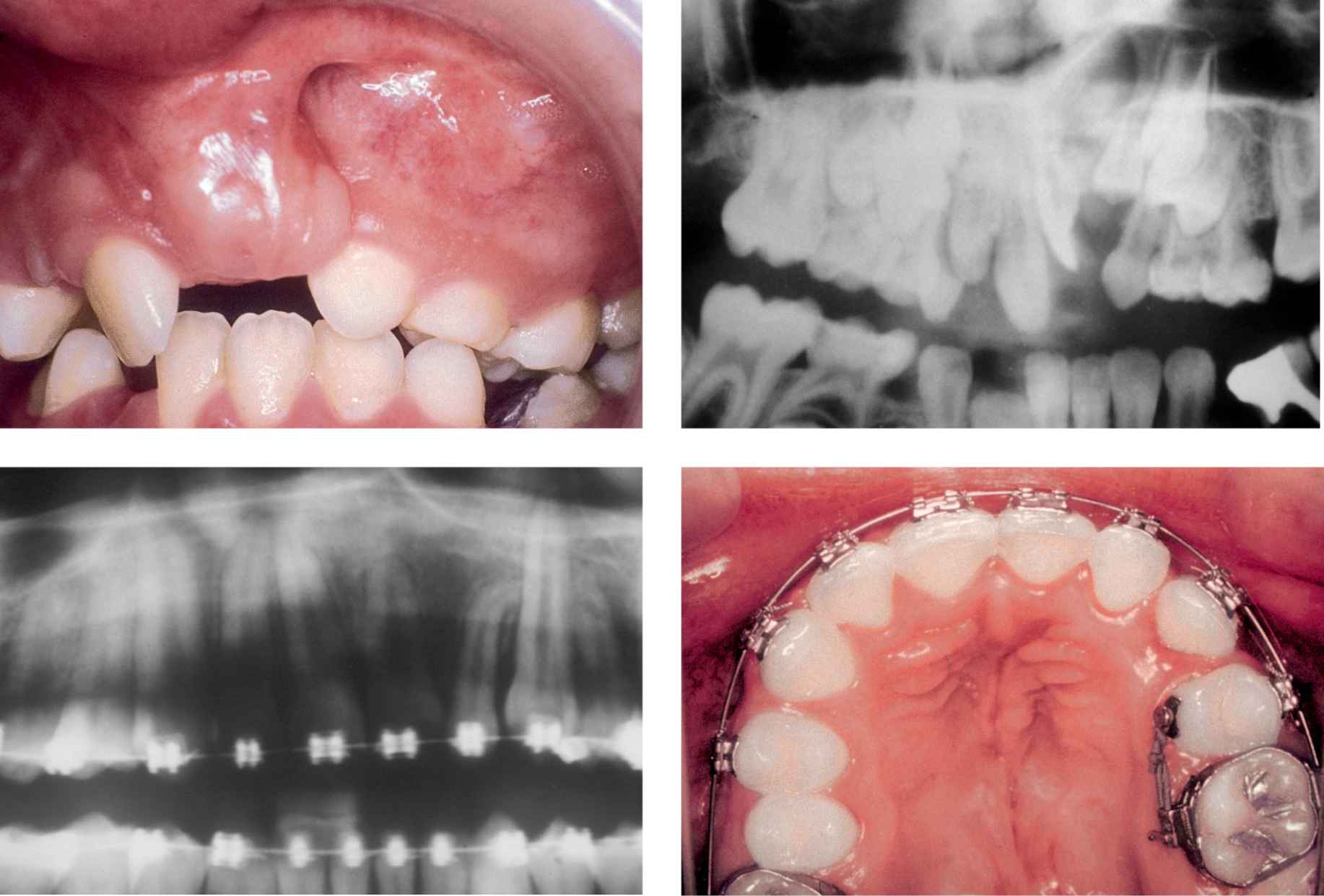
A few weeks after the operation the orthodontist can start moving the teeth towards the graft (Cases 8, 9).
CASE 8
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


