Fig. 35.1
Nasopharyngoscopic images of the velopharyngeal structures in a child with VPD. Left, velopharyngeal opening at rest. Right, persistent velopharyngeal gap during production of the /s/ sound (From 2nd Ed. Fig. 35.1)
To improve patient comfort with the procedure, topical anesthetic and/or nasal decongestant may be applied transnasally, although is not required. Application of a simple surgical lubricant to the external surface of the scope (avoiding the lens) also facilitates ease of transnasal insertion of the scope. Typically, nasopharyngoscopy is completed and/or interpreted by the speech pathologist and surgeon together and is videorecorded. High-resolution cameras (larger in diameter) are now available which offer improved image quality, magnification, field of view, and slow motion or frame-by-frame analysis; however, pediatric smaller-diameter nasopharyngoscopes tend to be better tolerated by younger patients. Once the scope is inserted transnasally through the middle or inferior meatus, the boundaries of the VP port should be visualized (i.e., anteriorly—soft palate, posteriorly—posterior pharyngeal wall/adenoid pad, laterally—lateral pharyngeal walls). The patient is then asked to engage in the production of a standard speech sample to assess VP closure during speech. The speech pathologist should select a speech sample focused on the patient’s accurately articulated words and sentences. A range of stimuli (i.e., different phonemic contexts, range of utterance length, and contrasting error vs. accurate productions) are useful for gaining an understanding of the underlying pathology; however, the most representative image of maximum attempted VP closure will be obtained during the accurately produced (articulated) oral pressure consonants in connected speech. Images obtained during compensatory articulation error production (e.g., glottal stops, nasal fricatives) have been shown to be associated with a lesser degree of attempted VP closure than accurate speech production (Henningsson and Isberg 1991). Overall, the resulting images obtained during speech will allow the speech pathologist and surgeon to determine the most appropriate type of treatment, as well as location, type, and size specifications for potential surgical intervention to improve VP closure for speech. Examples of speech stimuli include:
-
Pet a puppy.
-
Buy baby a bib.
-
Dad did it.
-
Tell Ted to try.
-
Go get a cookie for Kate.
-
Fifty-five fish.
-
Sissy sees the sky.
-
She likes to shop for shoes.
-
Chocolate chip cookies.
All of the above stimuli can be shortened if needed to adjust for patient speech proficiency or other articulation constraints. It is suggested that if the patient is only capable of accurate articulation at the single-word level, then multiple repetitions should be obtained in order to mimic the timing demands of natural VP closure (e.g., puppy-puppy-puppy). Additional stimuli including nasal consonants (e.g., My mom made muffins.) may be added to assess hyponasal speech or alternating oral-nasal contents (e.g., hamper hamper) to assess VP closure timing and coordination. The speech pathologist should carefully interpret the VP images in the context of the phonemic demands (i.e., oral vs. nasal, high pressure vs. low pressure) and accuracy of the sounds produced. Options for rating and measuring ratios of movement from the nasopharyngoscopy exam are outlined in Golding-Kushner et al. (1990).
In patients who demonstrate persistent or recurrent VPD after surgical management, careful intraoral and nasopharyngoscopic examination yields important diagnostic information. Pharyngeal flap integrity, width, port size, and position relative to the attempted level of velar closure should all be assessed. Occasionally, velar function may be impaired by the influence of a narrow flap that tethers the palate by virtue of being placed too low on the posterior pharyngeal wall. In such cases, the tethering flap should be surgically divided and the velopharynx reassessed. Similarly, the integrity, port size, and position of a previously constructed sphincter pharyngoplasty may yield important clues as to the nature of persistent VPD after surgical management.
MVVF is a valid alternative to nasopharyngoscopy for the assessment of VP function during speech, in most cases. MVVF typically requires a radiologist and speech pathologist to obtain and record the exam, and the images are later reviewed with the surgeon. Motion fluoroscopy records the movement of intraoral and velopharyngeal structures during speech from multiple angles (Skolnick 1970). Advantages of MVVF include more precise data regarding palatal length, pharyngeal depth, and relative size of upper airway anatomy, as well as information regarding the contribution of tongue movement to speech (or even to assisting VP closure). Image quality is best if liquid barium is instilled into the nasal cavity during the exam to coat the surfaces of the VP mechanism. At minimum, the lateral view is obtained, followed by frontal, base, and Towne’s views. Measurement and rating procedures for interpretation of MVVF are also reviewed in Golding-Kushner et al. (1990).
Overall, preoperative imaging of the velopharynx is essential to the accurate assessment of VPD and therefore to surgical planning. Such imaging serves as an essential adjunct to visual assessment of levator muscle orientation, velar integrity and function, and adenoid and tonsillar morphology. Occasionally, velopharyngeal dysfunction is found to result from attempted closure of the velum against an irregularly shaped adenoid pad. Similarly, marked tonsillar enlargement may also interfere with velopharyngeal closure (MacKenzie-Stepner et al. 1987a; Shprintzen et al. 1987). In such cases, consideration should be given to adenoidectomy or tonsillectomy, respectively, prior to reassessment of velopharyngeal function. In all cases, the extent, pattern, symmetry, and attempted level of velopharyngeal closure should be noted. When gap size is small and the levators are sagittal in position, Furlow palatoplasty may be sufficient treatment. In patients with larger gaps and/or transverse levator orientation, conventional wisdom suggests that closure pattern dictates the choice of procedure. Posterior pharyngeal flaps are theoretically best suited for those patients that demonstrate good lateral pharyngeal wall motion and sagittal closure patterns, whereas sphincter pharyngoplasty is theoretically best suited for those with limited lateral wall motion and coronal closure patterns. It should be noted, however, that, despite the intuitive basis for such an approach, there is little clinical evidence of its soundness.
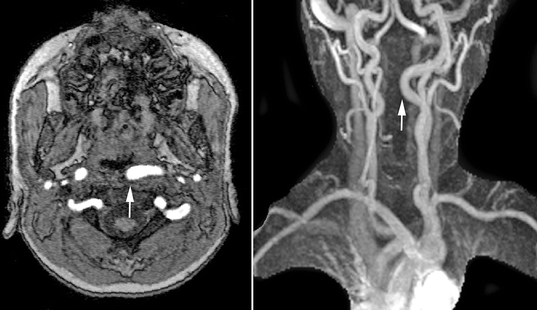
Magnetic resonance imaging should also be performed preoperatively in all patients with 22q11.2 deletion syndrome. One in five affected patients will demonstrate medial displacement of one or both internal carotid arteries (D’Antonio and Marsh 1987; MacKenzie-Stepner et al. 1987b; Mitnick et al. 1996; Ross et al. 1996), thus increasing the risk for arterial injury during posterior pharyngeal flap surgery or sphincter pharyngoplasty (Fig. 35.3). Arterial pulsations noted at the time of nasendoscopy may be transmitted through the surrounding soft tissues, rendering endoscopy alone an unreliable means of assessing carotid anatomy. Although some have suggested that carotid artery displacement should contraindicate pharyngoplasty, others have demonstrated that surgery can be safely performed in this setting (Witt et al. 1998b; Tatum et al. 2002). In many cases, the displaced vessel(s) will lateralize with neck extension at the time of surgery. When a displaced carotid artery can still be palpated beneath the posterior pharyngeal mucosa after positioning, the design of a pharyngeal flap can be modified, skewing the flap away from the displaced vessel. In all such cases, preoperative delineation of arterial anatomy plays an important role in surgical planning and informed consent.
35.2 Furlow Double-Opposing Z-Palatoplasty (Fig. 35.4)
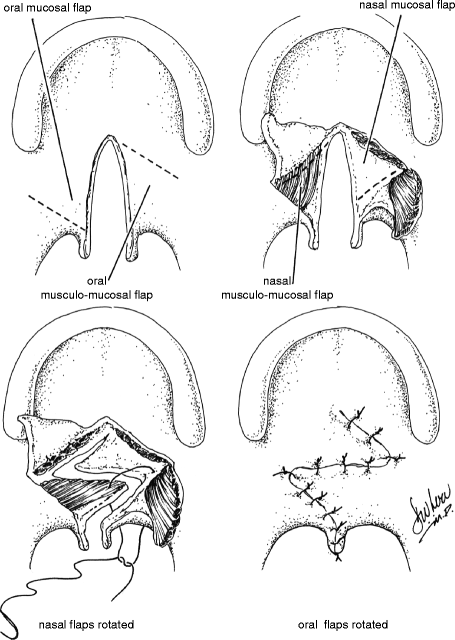
Despite its original description as a technique for primary repair of palatal clefts (Furlow 1978), the Furlow double-opposing Z-palatoplasty has increasingly become recognized as an effective procedure for the management of VPD in selected patients. Transposition of the posteriorly based flaps reorients the levator veli palatini muscle bundles and reconstructs the levator sling. It is for this reason, however, that the Furlow procedure should not be employed in patients who have already undergone complete intravelar veloplasty. The Z-plasty design provides for velar lengthening while preventing the cicatricial shortening that may be seen with straight-line velar repair. These features render the Furlow repair ideally suited for patients with VPD associated with unrepaired submucosal clefts and those with small-gap VPD following palate repair without intravelar veloplasty. The procedure should not be performed in patients that have previously undergone reconstruction of the levator sling.
The anatomy of the Z-plasty design is determined by the underlying palatal anatomy. The posteriorly based flaps of both the oral and nasal sides comprise both muscle and mucosa, whereas the anteriorly based flaps comprise mucosa alone. The Z-plasty incisions extend from the tip of the hamulus to the posterior edge of the hard palate at the cleft margin on one side and from the base of the uvula at the cleft margin to the tip of the hamulus on the other. Dissection must be carried out laterally to the hamulus in each side in order to ensure that the levator fibers are completely divided from the posterior edge of the hard palate. This is necessary for proper reconstruction and retropositioning of the levator sling with transposition of the posteriorly based flaps.
Several authors have confirmed the efficacy of the Furlow double-opposing Z-palatoplasty in the management of VPD. Hudson et al. (1995) reported that 85 % of patients with VPD following primary palatoplasty achieved normal resonance after Furlow conversion. Chen et al. (1994) reported gap size to be the most important factor in determining the success of Furlow palatoplasty in the management of VPD, with the majority of patients with a preoperative gap of 5 mm or less achieving velopharyngeal competence postoperatively. D’Antonio et al. (2000) reported normal resonance after conversion to Furlow palatoplasty in 75 % of selected patients with post-palatoplasty VPD. All patients in their series demonstrated incomplete levator retropositioning, good velar motion, and a small velopharyngeal gap preoperatively.
As noted above, the Furlow procedure is ideally suited to those patients with nonsyndromic submucosal cleft palate and VPD. As in patients with repaired overt clefts, success rates appear to depend primarily on gap size. Seagle et al. (1999) reported that 83 % of patients with VPD associated with submucosal cleft palate demonstrated velopharyngeal competence after Furlow Z-plasty, with successful outcomes observed most frequently in patients with a gap size under 8 mm. Chen et al. (1996) noted that VP competence was achieved in 97 % of patients with submucosal clefts and gaps of 5 mm or less. Studies have confirmed that the Furlow Z-plasty produces a variable degree of palatal lengthening (D’Antonio et al. 2000). As noted above, however, the geometry of the Z-plasty incisions is determined by the underlying palatal anatomy. It stands to reason, therefore, that the success of the procedure is inevitably linked to gap size and to the extent of velar lengthening that can be achieved given the underlying palatal anatomy.
Bleeding, oronasal fistula formation, and upper airway obstruction have all been reported following Furlow palatoplasty. By virtue of the Z-plasty design, the technique achieves palatal lengthening at the expense of velar width, thereby creating a potential increase in tension at the suture line. Fistula formation can be minimized, however, by reducing tension through the liberal use of lateral relaxing incisions. Although mild upper airway obstruction has been documented following Furlow palatoplasty, such has been noted to resolve in nearly all patients within 3 months of surgery (Liao et al. 2003). Moreover, patients who have undergone Furlow palatoplasty demonstrate a significantly lower incidence and severity of upper airway obstruction 6 or more months postoperatively than do those patients who have undergone posterior pharyngeal flap surgery (Liao et al. 2004).
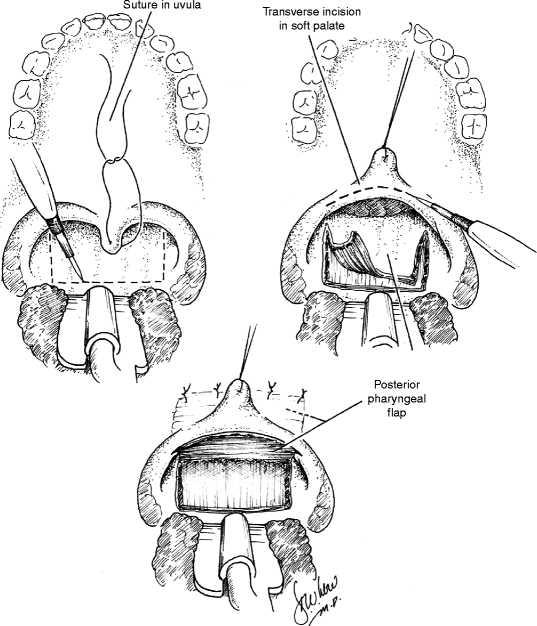
35.3 Posterior Pharyngeal Flap (Fig. 35.5)
In 1865, Passavant first described the surgical management of VPD by attachment of the velum to the posterior pharynx (Passavant 1865). Schoenborn described the use of an inferiorly-based posterior pharyngeal flap in 1875 and of a superiorly-based flap a decade later (Schoenborn 1875, 1886). Pharyngeal flap surgery was popularized in the United States by Padgett in the 1930s (Padgett 1930). For much of the twentieth century, the pharyngeal flap remained the workhorse of VPD surgery. With the addition of several modifications, the technique remains widely popular today.
The posterior pharyngeal flap acts as a static central obturator of the velopharynx, relying upon lateral pharyngeal wall motion to close the lateral ports during sound production. The procedure is best suited, therefore, for management of VPD in the patient with a sagittal or sphincteric closure pattern and a persistent central gap. Laterally based, inferiorly based, and superiorly based flaps have all been described, though the last remains the most widely used design today. Optimization of surgical outcome relies upon careful placement of the flap at the attempted level of velar contact with the posterior pharyngeal wall, as determined by preoperative imaging. As noted above, pharyngeal flaps that have been designed too low or that have migrated inferiorly due to cicatricial changes may tether the velum and restrict velopharyngeal closure.
Just as for flap position, the width of the pharyngeal flap should be tailored to each patient’s needs as determined by preoperative assessment. Patients with large gaps and relatively poor lateral wall motion may require wider flaps in order to achieve velopharyngeal competence than those with smaller gaps and more robust lateral wall motion. Flap width is dependent not only on the breadth of the flap design but also on the breadth of flap inset into the soft palate. Inset may be achieved by dividing the velum in the midline or through the use of a transverse incision across the posterior velum, the latter technique allowing for greater flexibility in establishing flap width and, conversely, lateral port dimension (Argamaso 1995). The tendency for flaps to narrow or “tube” over time may be minimized by the creation of mucosal lining flaps from the posterior velum or through the use of short, broad pharyngeal flaps. In all cases, flap design must be balanced against the risk of inducing postoperative upper airway obstruction, as wider flaps carry a higher risk of postoperative sleep apnea.
Careful surgical planning and individualization of flap design and inset are essential to achieving successful outcomes in pharyngeal flap surgery. Canady et al. (2003) reported their 10-year experience with pharyngeal flap surgery at the University of Iowa. Success, defined as normal or near normal resonance, was achieved in 78 % of patients. Argamaso (1995) reported elimination of hypernasality in 96 % of patients following pharyngeal flap surgery. Similarly, borderline or normal velopharyngeal function was observed postoperatively by Sullivan et al. in 97 % of patients (Sullivan et al. 2010). Studies have demonstrated the results of pharyngeal flap surgery to be durable, with stable results noted more than a decade after surgical treatment (Cable et al. 2004).
The reported complication rate for pharyngeal flap surgery ranges from 6.3 to 19.5 % (Valnicek et al. 1994; Fraulin et al. 1998; Hofer et al. 2002). The operation may be complicated by hemorrhage, flap dehiscence, hyponasality, persistent VPD, and nasal airway obstruction. Airway compromise has been reported to result in a small number of deaths after posterior pharyngeal flap surgery. Complications may be increased by association with limited operator experience, associated medical conditions, and concurrent surgical procedures. Of all complications, upper airway compromise is the most common. Due to postoperative edema, nearly all patients demonstrate some transient nasal airway obstruction following posterior pharyngeal flap surgery, and careful monitoring during the immediate postoperative period is therefore essential. In all but a small percentage of patients, nocturnal airway obstruction resolves within several months of surgery. Syndromic patients and those with a history of Pierre Robin sequence often present with associated functional or anatomic airway anomalies and may therefore be at greater risk for persistent upper airway obstruction after surgery (Wells et al. 1999; Abramson et al. 1997). The presence of tonsillar hypertrophy should alert the surgeon to an increased risk of sleep apnea following creation of a pharyngeal flap (Ysunza et al. 1993). In such cases, tonsillectomy should be performed prior to velopharyngeal imaging and subsequent pharyngeal flap surgery.
Persistent VPD following posterior pharyngeal flap surgery may result from inappropriate use of the procedure (i.e., poor lateral wall motion), from poor surgical design (i.e., a flap that is too low or too narrow), or from cicatricial alterations in flap location or dimension. In all cases, the treatment team must allow adequate time for resolution of edema and for scar maturation prior to consideration of surgical revision, usually a period of 12 months. Persistent VPD demands the same precision in diagnosis and management as does the initial condition. In all cases, nasendoscopic visualization is essential to assessment of the altered velopharyngeal valve, and surgical management should be tailored to the functional and anatomic needs so identified.
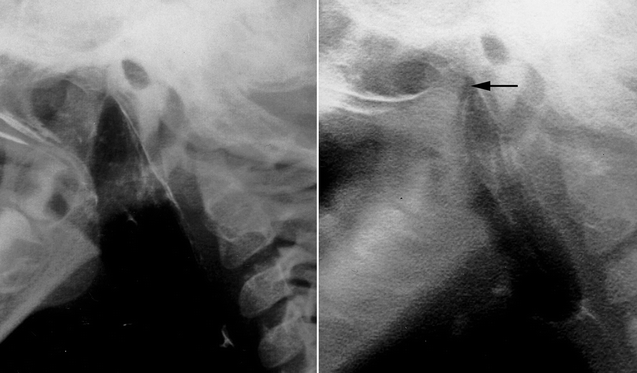
35.4 Sphincter Pharyngoplasty (Fig. 35.6)
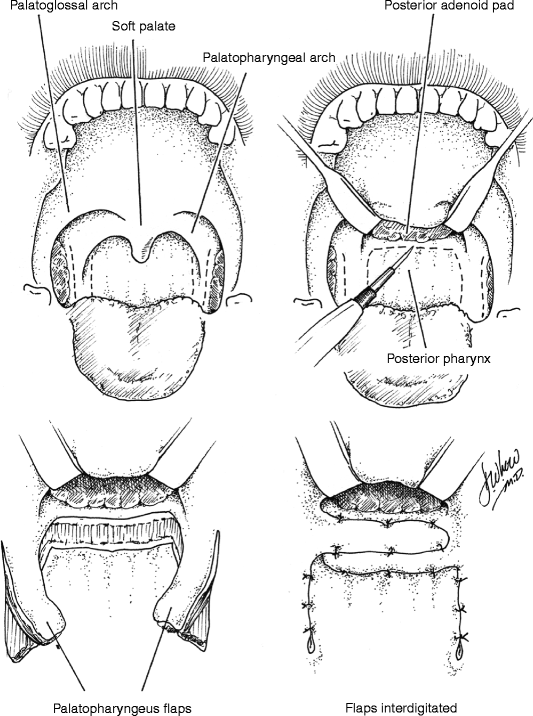
Fig. 35.6
Sphincter pharyngoplasty (From Murphy and Scambler (2005), with permission. From 2nd Ed. Fig. 35.5)
In 1950, Hynes first described the treatment of VPD by transposition of myomucosal flaps containing the salpingopharyngeus muscles (Hynes 1950). He later modified the technique, constructing a sphincter containing the palatopharyngeus muscles (Hynes 1953). The procedure’s success was attributed to narrowing of the velopharynx and augmentation of the posterior pharyngeal wall with bulky, often contractile flaps (Hynes 1967). The concept of dynamic sphincter pharyngoplasty was later championed by Orticochea (1968). Jackson later modified the Orticochea’s procedure and described the technique of sphincter pharyngoplasty that is widely utilized today (Jackson and Silverton 1977).
In the construction of a sphincter pharyngoplasty, vertical incisions are made at the junction of each posterior tonsillar pillar with the adjacent tonsillar fossa. The longitudinally oriented palatopharyngeus muscles are dissected and included in the superiorly based flaps in order to maximize bulk and contractility. Vertical incisions are then made at the junction of the posterior pillars with the posterior pharyngeal wall, the inferior aspect of each flap is then divided, and the flap elevated. The flaps are then rotated medially and inset into a transverse incision in the posterior pharyngeal mucosa that joins the superior aspect of the medial incisions. Riski et al. (1984) have written that the level of inset is critical to success of the procedure and should correspond to the attempted level of velar contact as determined by preoperative imaging. Central port size may be controlled by altering the extent of flap overlap on the posterior pharyngeal wall and should be individualized to the anatomic and functional needs of each patient. Several studies have shown that not all sphincters demonstrate contractility (Kawamoto 1995; Witt et al. 1998a). Port size and flap bulk may therefore play critical roles in achieving velopharyngeal competence.
Sphincter pharyngoplasty successfully manages VPD in the majority of carefully selected patients. Retrospective reviews by Shewmake et al. (1992) and by Mount and Marsh (2002) showed that velopharyngeal competence was achieved in 85 and 72 % of patients, respectively. Riski et al. (1992) reported that 78 % of 139 patients demonstrated resolution of hypernasality and normalization of pressure-flow measurements following sphincter pharyngoplasty. Poor results were associated with improper placement of the sphincter on the posterior pharyngeal wall. Reports by Witt et al. (1998c) and Losken et al. (2003) cite pharyngoplasty revision rates for persistent VPD of 16 and 12.8 %, respectively. In these series, poor outcomes were associated with dehiscence, syndromic diagnoses, and greater preoperative nasalence scores.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses