Fig. 9.1
(a) An X-ray figure showing proximal cavities in premolars. (b) An old silver amalgam restoration and fissures. (c) Occlusal restoration with caries-infiltrated fissures
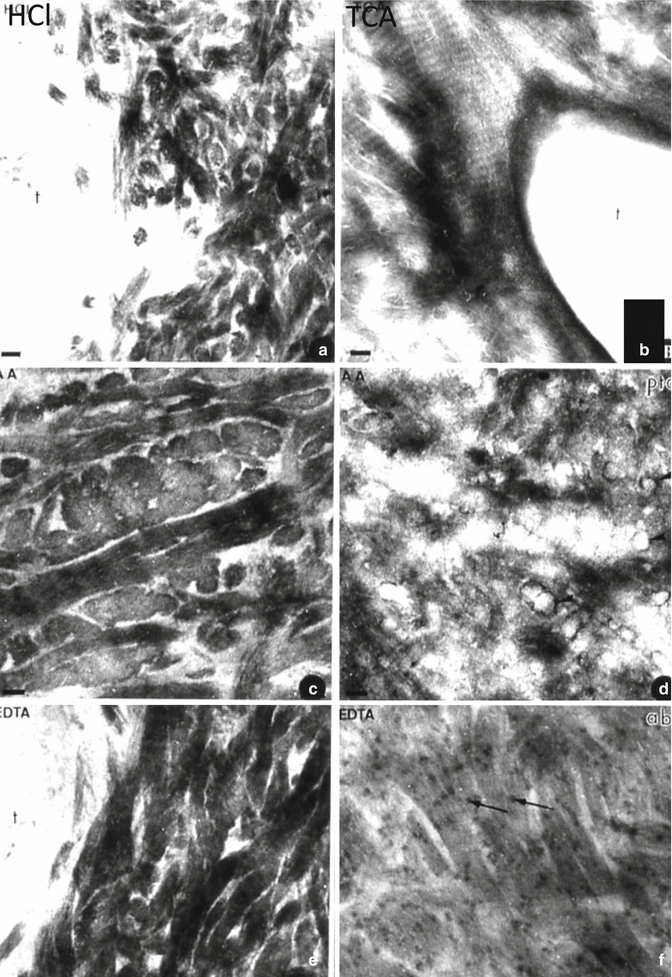
Fig. 9.2
After acid or chelator demineralization, intercollagenous spaces are widely open. After hydrochloric acid (HCl) (a), trichloroacetic acid (TCA) (b), acetic acid (AA) (c, d), or ethylendiamine tertracetic acid (EDTA) (e, f) demineralization, intercollagenous spaces are widely open
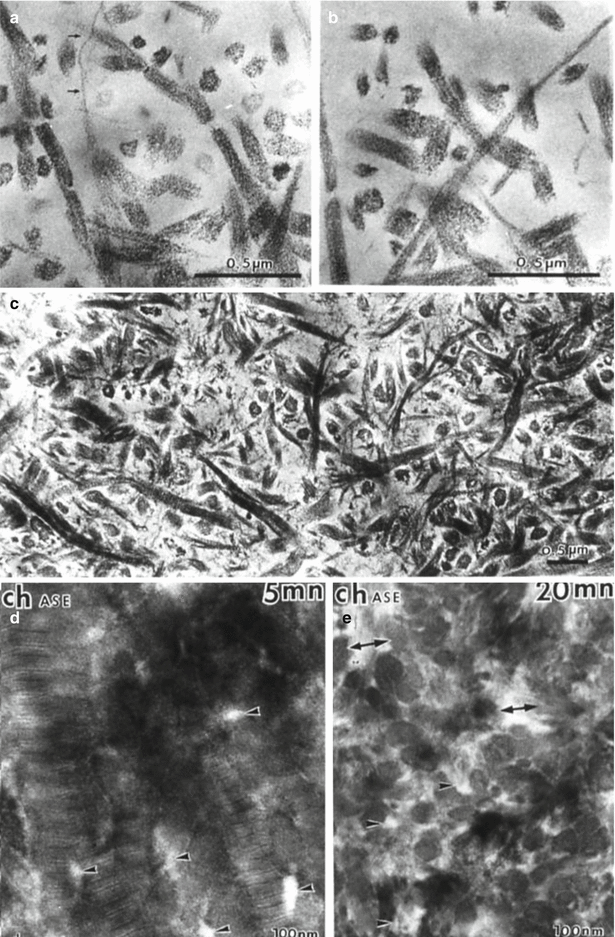
Fig. 9.3
(a–c) Collagen is broken in ¼ and ¾ peptides after action of a bacterial collagenase. (d, e) Effects of a chondroitinase A–C after 5 min (d) and 20 min (e). Arrows: collagen fibrils. Arrowheads and double headed arrows: intercollagen spaces enlarged after 20 min digestion by the enzyme
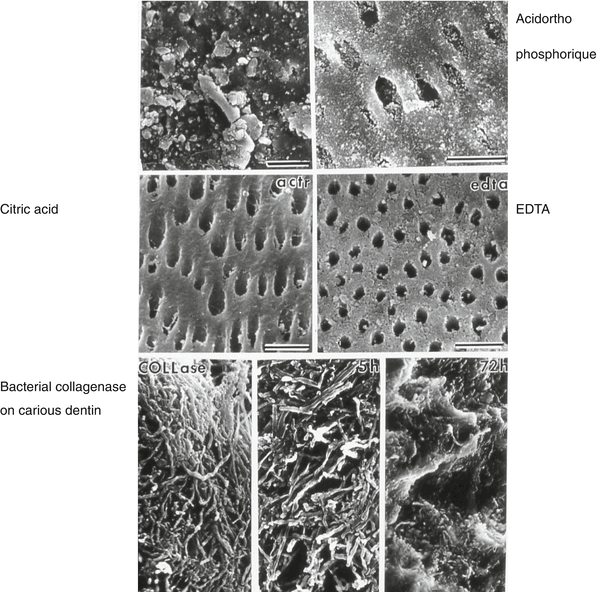
Fig. 9.4
Outer surface cleaning by orthophosphoric acid (upper figures), citric acid, and EDTA. Lower figures: action of a bacterial collagenase on carious dentin
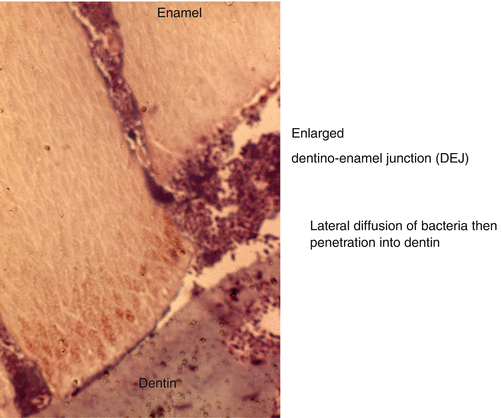
Fig. 9.5
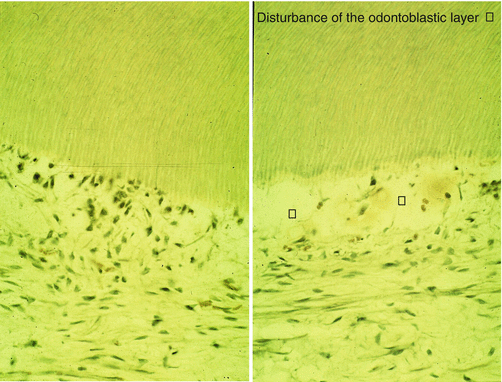
Carious lesion penetrating up to the dentinoenamel junction (DEJ). This is followed by a lateral diffusion of bacteria destroying the superficial mantle dentin
Caries may be superficial, located near the dentinoenamel junction (peripheral excavated and hard dentin), or located in the middle of the dentin layer (central demineralized dentin before the final excavation), or located in the inner part, near the pulp (central dentin after the final excavation) (Bjørndal et al. 1997). Therefore the lesion can be either superficial or degrade the deepest part of the coronal dentin. The density of the tubules (number of tubules/mm2) and/or their diameter appears to be correlated to this anatomical distribution. The pulp chamber ceiling presents the higher tubule density. The rate of development of carious lesions is due, in part, to their tubule density; a high density implicates a rapid spread (Figs. 9.2, 9.3, and 9.4).
Tubule density in the middle layer of the crown was 18.243 ± 3.845 in human deciduous vs. human permanent dentin. In the deep layer, the mean value was 24.162 ± 5. 338. In the permanent dentin, the number of tubules per mm2 was 21.343 ± 7.290 in the deep layer and 18.781 ± 5.855 in the middle layer (Schilke et al. 2000). Variations of tubule density have been noticed. It comes out that in the superficial dentin, the tubule density appears to be 17.433 ± 1.370, in the outer dentin 18.075 ± 2.415, in the intermediate layer 20.433 ± 2.568, and in the deep dentin 26.391 ± 6.605 (Koutsi et al. 1994). The mean numbers of tubules at any given age within coronal, cervical, and mid-rot dentin are quite similar (approximately 44.243, 42.360, and 39.010 mm2, respectively) (Carrigan et al. 1984). However, significantly fewer dentinal tubules are found in apical dentin (approximately 8.190 mm2). Differences reported between publications were striking, with a variation of 40.000–51.000 tubules per mm2. The number of dentinal tubules per mm2 varies from 15.000 μm at the dentinoenamel junction to 45.000 μm near the pulp (Garberoglio and Brännström 1976). In general, the tubule density increases in relation with the dentin depth.
The diameter of the tubules was seen to vary depending the species and the location in space and time. In young people, the pulp chamber floor presents high tubule diameter, with a dimension of dentinal tubule openings decreasing with age (Kontakiotis et al. 2015). In deciduous dentin, the diameter of the tubules varied between the deep layer (2.82 ± 0.28) and middle layer (2.55 ± 0.16). In permanent dentin, the diameter varied between the deep layer (2.90 ± 0.22) and the middle layer (2.65 ± 0.19) (Schilke et al. 2000). The tubule diameter varies between the superficial 0.96, outer 1.08, intermediate 1.10, and deep layers 1.29 (Koutsi et al. 1994). The tubule diameter increases together with the dentin depth.
Human carious lesions imply collagen degradation. This is under the control of members of the metalloproteinase family (MMP-1, MMP-2, MMP-8, MMP-13, and MMP-14), human neutrophil elastase, and cysteine proteinases. MMP-2 and MMP-9, also named gelatinases A and B, cut the collagen fibers into two parts (1/4th and 3/4th) fragments. MMP-2 and MMP-9 can remove telopeptides from the ends of helical collagen, but only MMP-8 is a true collagenase that can cleave the collagen in 2 large fragments. Cathepsin K cleaves both helical and telopeptide regions. Expression in sound, caries-affected and caries-infected dentin. Cysteine cathepsins degrade type I collagen, laminin, fibronectin, and proteoglycans. Cathepsins B and L cleave the non-helical telopeptide extensions of collagens, whereas cathepsin K cleaves the collagen at the triple helical region. Caries stimulates MMP-2 expression in sound, caries-affected and caries-infected dentin. Cysteine cathepsins degrade type I collagen, laminin, fibronectin, and proteoglycans. Cathepsins B and L cleave the non-helical telopeptides extensions of collagens, whereas cathepsin K cleaves the collagen at the triple helical region (Nascimento et al. 2011).
Dentin lesions may be classified in superficial lesion (depth up to 2–3 mm), deep lesion (depth up to 3–5 mm), and deep lesions (with pulp exposure) (Figs. 9.6, 9.7, 9.8, 9.9, and 9.10).