Introduction
The purpose of this study was to review available evidence on the efficacy of various oral appliances on subjectively perceived symptoms of obstructive sleep apnea syndrome.
Methods
A search of 4 databases was carried out. Articles were initially selected based on their titles or abstracts. Full articles were then retrieved and further scrutinized according to predetermined criteria. Reference lists of selected articles were searched for any missed publications. The finally selected articles were methodologically evaluated.
Results
Of an initial 1475 references, 14 studies were randomized controlled trials, which formed the basis of this review. Mandibular advancement devices (MADs) were compared with either inactive appliances (6 studies) or MADs with different design features (8 studies). In comparison with inactive appliances, the majority of studies showed improved subjective outcomes with MADs, suggesting that mandibular advancement is a crucial design feature of oral appliance therapy for obstructive sleep apnea syndrome.
Conclusions
There is no 1 MAD design that most effectively influences subjectively perceived treatment efficacy, but efficacy depends on many factors including materials and method used for fabrication, type of MAD (monoblock or Twin-block), and the degree of protrusion (sagittal and vertical). This review highlights the absence of universally agreed subjective assessment tools and health-related quality of life outcomes in the literature today. Future trials of MAD designs need to assess subjective efficacy with agreed standardized tools and health-related quality of life measures to guide clinical practicitioners about which design might be most effective in the treatment of obstructive sleep apnea syndrome with oral appliances.
Obstructive sleep apnea syndrome (OSAS) is the most common organic sleep disorder and is increasingly recognized as a serious public health issue. Its prevalence worldwide is estimated at 3% to 7% in men and 2% to 5% in women. Apart from having serious consequences for a patient’s physiologic health, untreated OSAS causes particular morbidity in terms of the health-related quality of life (HRQL) a patient experiences. The inability to sleep sufficiently at night causes excessive daytime somnolence, which impacts the ability to function at an optimum level, can lead to depression, contributes to traffic accidents, and disrupts social relationships.
The latest Cochrane review on oral appliances (OAs) for OSAS highlighted that they are increasingly recognized as a suitable and effective treatment option. Mandibular advancement devices (MADs) are the most commonly prescribed OAs in the treatment of OSAS. Although MADs are more effective than other types of OAs in treating OSAS, it has been suggested that design features of the various appliances can have an impact on treatment efficacy. The Cochrane review detailed some evidence that MADs improve subjective sleepiness compared with placebo appliances. A review by Ahrens et al evaluated the efficacy of various MAD designs on patients’ objective polysomnographic outcomes; however, it is unclear how the different design features of the various MADs impact patients’ subjective evaluations of treatment effect. Understanding whether a type or design of MAD is most effective in the subjective treatment of OSAS is imperative in informing patient-centered evidence-based practice. Therefore, in this review, we aimed to summarize the evidence on the efficacy of differently designed MADs on the subjective patient-centered outcome measures of OSAS.
Material and methods
To identify studies relevant to OA treatment for OSAS, a computerized database search was carried out by using MEDLINE, EMBASE, Cinahl, and the Cochrane Library ( Table I ). No language limitations were set, and the search was limited to human studies. If articles contained the search thesaurus anywhere, they were selected to constitute a list of potentially eligible studies to be included in this review.
| Obstructive sleep apnoea syndrome part | |
| 1 | Obstructive sleep apnea (MeSH word) |
| 2 | Obstructive sleep (apnoea or apnea) |
| 3 | Sleep (breathing disorder ∗ or respiratory disorder ∗ ) |
| 4 | 1 OR 2 OR 3 |
| Oral appliance part | |
| 5 | Orthodontic appliances (MeSH word) |
| 6 | Oral (device ∗ or appliance ∗ or splint) |
| 7 | Dental (device ∗ or appliance ∗ or splint) |
| 8 | Orthodontic ∗ (device ∗ or appliance ∗ or splint) |
| 9 | Mandib ∗ advancement ∗ |
| Final search syntax | |
| 10 | 4 AND (5 or 6 or 7 or 8 or 9) |
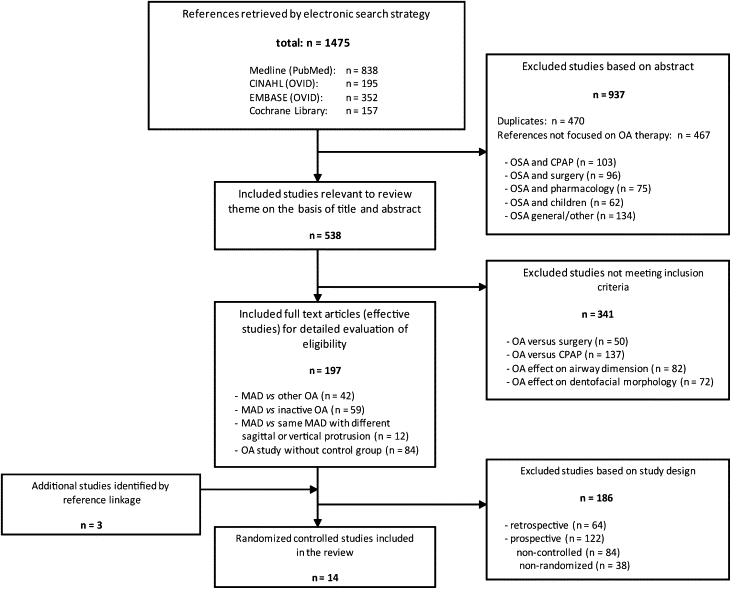
Titles and abstracts of study references on this list were reviewed by 2 independent researchers, who then determined whether they were relevant to the theme of this review: studies exclusively focusing on OA therapy for OSAS treatment ( Fig ). If the researchers disagreed about which articles were relevant, consensus was reached by discussion. To select articles that lent themselves to assessing the impact of appliance design on subjective treatment efficacy, 3 inclusion criteria were set. Only studies that investigated MAD vs other OA, MAD vs inactive OA, or the same MAD but with varying degrees of mandibular advancement or vertical bite opening were selected to remain on the list of potential studies for this review. The full texts of these studies were then obtained, and the reference lists were searched manually for additional relevant publications (reference linkage). All studies were methodologically appraised according to the American Association of Sleep Medicine’s levels of evidence ( Table II ) to identify “effective” articles.
| Evidence level | Study design |
|---|---|
| I | Randomized well-designed trials with low alpha and beta error ∗ |
| II | Randomized trials with high alpha and beta error ∗ |
| III | Nonrandomized concurrently controlled studies |
| IV | Nonrandomized historically controlled studies |
| V | Case series |
∗ Alpha (type I error) refers to the probability that the null hypothesis is rejected when in fact it is true (generally acceptable at 5% or less, or P <0.05). Beta (type II error) refers to the probability that the null hypothesis is mistakenly accepted when in fact it is false (generally trials accept a beta error of 0.20). The estimation of type II error is generally the result of a power analysis. The power analysis takes into account the variability and the effect size to determine whether the sample size is adequate to find a difference in means when it is present (power is generally acceptable at 80%-90%).
Results
Initially, 1475 references ( Fig ) were retrieved from the primary database searches; among them, there were 470 duplicate references. An additional 467 references were excluded because they were not relevant for this review. Of the remaining 538 study references, a further 341 were excluded because they did not meet the criteria for inclusion ( Fig ). Full texts of the remaining 197 references were obtained, and an additional 3 articles were identified as potentially relevant by reference linkage. Among these 200 studies, 6 could be categorized as evidence level I, and 8 studies as level II of evidence. Fifty-nine studies reached evidence level III; 3 studies, level IV; and 124 studies, level V. Based on this classification of evidence, the 14 levels I and II randomized controlled trials were finally selected as the basis of this review. These studies were grouped according to the following outcome measures: (1) MAD vs inactive control OA, (2) studies comparing 1-piece MADs with 1-piece MADs, (3) studies comparing 2-piece MADs with 2-piece MADs, and (4) studies comparing 1-piece MADs with 2-piece MADs.
To assess patients’ subjective daytime sleepiness, all but 2 studies used the standardized and disease-specific Epworth sleepiness scale (ESS). To pool the data, 2 studies had to be excluded because only median values were reported. The ESS scores across the remaining 12 studies improved with the use of MADs and showed a mean reduction from 12.0 to 7.9. Gauthier et al used a fatigue severity scale, and Bloch et al used the sleep disorder questionnaire and a modified version of a sleep symptom questionnaire.
Four trials assessed patients’ HRQL by standardized tools. These were either generic—the medical outcome survey short form (SF-36)—or sleep-specific—the functional outcomes of sleep questionnaire (FOSQ). One study used both assessment tools.
Subjective treatment efficacy was assessed by nonstandardized (in-house) questionnaires or a visual analog scale (VAS) by 6 studies. Treatment compliance, treatment satisfaction, appliance preference, side effects, snoring, and sleep quality were generally self-reported by questionnaire. Across all studies, symptoms were reported to be mild to moderate and temporary, with temporomandibular joint pain, muscular, dental and gingival discomfort, dry mouth, and excessive salivation as the most common. Treatment compliance was generally high (dropout rates, 0%-26%). Average usage of individual appliances could not be calculated because this was reported differently across the reviewed studies, with some reporting nightly usage per week and others hourly usage per night. The studies’ durations ranged from 2 weeks to 12 months, and the sample sizes of the target study populations varied considerably from 16 to 93 subjects, but most studies specified a sample size between 20 and 30 ( Tables III and VI ).
| Patients (n) | Oral appliance | Advancement | Assessment tool | Result | Statistical | Subjective treatment satisfaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Baseline | Complete | (OA) | Sagittal | Vertical | used | Pretreatment | Posttreatment | significance | ||
| Blanco et al | 24 | 15 | A: MAD (1-piece soft elastic silicone positioner) |
75% of maximum protrusion |
5 mm | ESS | MAD | 14.7 ± 5.1 | 5.1 ± 1.9 | P <0.05 | Not reported |
| Inactive OA | 16.3 ± 2.5 | 13.6 ± 6.7 | NS | ||||||||
| B: inactive OA | – | – | SF-36 | ||||||||
| Physical | MAD | 70.7 (16.4) | 74.1 (18.4) | NS | |||||||
| functioning | Inactive OA | 71.5 (20.7) | 78.8 (19.1) | NS | |||||||
| Role | MAD | 83.4 (30.2) | 87.5 (30.6) | NS | |||||||
| physiological | Inactive OA | 81.2 (34.7) | 87.5 (35.6) | NS | |||||||
| Role emotional | MAD | 81.0 (37.7) | 77.7 (46.6) | NS | |||||||
| Inactive OA | 80.0 (29.9) | 87.5 (12.5) | NS | ||||||||
| Social | MAD: | 78.3 (13.6) | 78.2 (12.4) | NS | |||||||
| functioning | Inactive OA | 81.3 (18.8) | 79.4 (26.9) | NS | |||||||
| Mental health | MAD | 60.1 (19.3) | 59.4 (19.2) | NS | |||||||
| Inactive OA | 52.0 (15.7) | 56.0 (18.0) | NS | ||||||||
| Energy | MAD | 49.3 (18.8) | 50.7 (8.4) | NS | |||||||
| Inactive OA | 55.2 (12.2) | 56.2 (19.2) | NS | ||||||||
| Bodily pain | MAD | 70.3 (38.7) | 67.0 (21.3) | NS | |||||||
| Inactive OA | 65.3 (37.4) | 65.5 (19.2) | NS | ||||||||
| General health | MAD | 60.7 (22.0) | 61.0 (20.7) | NS | |||||||
| perception | Inactive OA | 57.4 (6.8) | 58.4 (10.5) | NS | |||||||
| FOSQ | MAD | 78.1 (22.6) | 99.3 (14.4) | P <0.05 | |||||||
| Inactive OA | 83.7 (20.8) | 82.3 (13.9) | NS | ||||||||
| General sleep | MAD | 15.4 ±1.9 | 10.1 ± 3.2 | P <0.05 | |||||||
| questionnaire: | Inactive OA | 14.4 ± 3.0 | 14.6 ± 1.7 | NS | |||||||
| snoring levels | |||||||||||
| Gotsopoulos et al |
73 | 73 | A: MAD (custom-made 2-piece) |
Mean 80% ± 9% (range, 50%-95%) of maximum protrusion mean 7 ± 2 mm (range, 3-13 mm) |
3-4 mm | ESS | MAD | 11 ± 5 | 7 ± 1 | MAD vs inactive: | MAD produced normal ESS in 60 (82%) patients compared with 45 (62%) on inactive treatment ( P <0.01) |
| Inactive OA | 9 ± 1 | P <0.001 | |||||||||
| B: inactive OA | – | – | Symptom questionnaire: Snoring | MAD | Not reported | 207 ± 20 | MAD vs inactive: | Treatment with MAD was significantly more satisfactory ( P <0.001) | |||
| frequency | Inactive OA | Not reported | 366 ± 21 | P <0.001 | |||||||
| Snoring loudness | MAD | Not reported | 48 ± 1 | MAD vs inactive: | |||||||
| Inactive OA | Not reported | 51 ± 1 | P <0.001 | ||||||||
| Improved sleep quality | MAD | Not reported | Not reported | MAD vs inactive: | |||||||
| Inactive OA | Not reported | Not reported | P <0.001 | ||||||||
| Hans et al | 24 | 18 | A: MAD (commercial thermoplastic 1-piece SnoreGuard) |
6-8 mm | 8 mm | ESS | MAD | 12.0 ± 3.9 | 8.2 ± 4.0 | P <0.033 | Not reported |
| B: inactive OA | – | 1 mm | Inactive OA | 13.0 ± 4.5 | 12.5 ± 5.7 | NS MAD vs inactive: NS |
|||||
| Johnston et al | 21 | 20 | A: MAD (customized 1-piece) | 75% of maximum protrusion mean, 5.7 mm (range, 4-9 mm) |
4 mm inter-incisal | ESS | MAD | 13.90 ± 6.39 | 11.6 ± 6.7 | MAD vs inactive: | MAD produced posttreatment ESS of ≤10/h in 45% of patients; however, 60% of those showed ESS of ≤10/h at baseline |
| Inactive OA | 12.6 ± 6.3 | NS | |||||||||
| B: inactive OA | – | 1.5 mm | Sleep questionnaire | MAD | Not reported | 2.58 ± 1.26 | MAD vs inactive: | ||||
| Inactive OA | Not reported | 3.16 ± 1.38 | NS | ||||||||
| Mehta et al | 28 | 24 | A: MAD (custom-made 2-piece) | Not reported | ESS | MAD | 10.1 ± 1.1 | 3.9 ± 0.6 | Posttreatment vs pretreatment: | Majority of patients reported substantial improvements in snoring (n =17, 70%) and sleep quality (n = 22, 91%) | |
| Mean, 78% (range, 63%-89%) maximum protrusion mean, 7.5 ± 1.8 mm (range, 5-11.5 mm) | P <0.01 | ||||||||||
| Inactive OA | Not reported | ||||||||||
| B: inactive OA | – | – | Questionnaire: Snoring, sleep quality | MAD | Not reported | Not reported | Not reported | ||||
| Inactive OA | Not reported | Not reported | Not reported | ||||||||
| Petri et al | 93 | 81 | A: MAD (custom-made 1-piece acrylic) |
Mean protrusion, | 5 mm in front | ESS | Posttreatment vs pretreatment: | Not reported | |||
| 74% (range, 64%-85%) |
MAD | 11.7 ± 4.3 | 8.4 ± 4.3 | P <0.001 | |||||||
| Inactive OA | 10.8 ± 4.6 | 9.6 ± 4.2 | P = 0.05 | ||||||||
| No intervention | 10.7 ± 4.6 | 10 ± 4.8 | NS | ||||||||
| MAD Inactive OA No intervention |
8.4 ± 4.3 9.6 ± 4.3 10.0 ± 4.8 |
Difference in means between groups: 0.044 † | |||||||||
| B: inactive OA | – | Not reported | SF-36: | Posttreatment vs pretreatment: | |||||||
| MCS ∗ | MAD Inactive OA No intervention |
47.2 ± 8.5 48.8 ± 10.0 50.2 ± 8.9 |
51.1 ± 8.0 49.8 ± 8.5 51.2 ± 7.8 |
P = 0.039 NS NS |
|||||||
| C: no intervention | – | – | PCS ∗ | MAD Inactive OA No intervention |
45.5 ± 9.5 48.1 ± 9.2 46.6 ± 9.6 |
46.5 ± 8.0 47.5 ± 11.2 47.3 ± 8.7 |
NS NS NS |
||||
| General health ∗ | MAD Inactive OA No intervention |
60.7 ± 21.9 66.6 ± 22.1 62.7 ± 19.8 |
66.7 ± 20.8 66.0 ± 22.1 67.0 ± 19.5 |
P = 0.011 NS NS |
|||||||
| Mental health ∗ | MAD Inactive OA No intervention |
71.0 ± 14.7 78.4 ± 19.5 79.6 ± 15.2 |
76.4 ± 13.8 80.4 ± 12.9 79.0 ± 15.4 |
P = 0.016 NS NS |
|||||||
| Vitality ∗ | MAD Inactive OA No intervention |
41.5 ± 23.4 47.8 ± 26.7 48.1 ± 24.3 |
59.4 ± 24.7 47.0 ± 26.4 51.3 ± 23.4 |
P <0.001 NS NS |
|||||||
| MAD Inactive OA No intervention |
59.4 ± 24.7 47.0 ± 26.4 51.3 ± 23.4 |
Difference in means between groups: 0.001 † | |||||||||
∗ Mental component summary ( MCS ) and physical component summary ( PCS ) and 3 domains, general health, mental health and vitality in SF-36
† Only vitality domain and ESS score differed significantly between intervention groups, with means in MAD group significantly different from means in inactive OA and no intervention groups (means of latter 2 groups did not differ significantly).
| Patients (n) | Oral appliance | Advancement | Assessment tool | Result | Statistical | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Baseline | Complete | (OA) | Sagittal | Vertical | used | Pretreatment | Posttreatment | significance | Subjective treatment satisfaction | |
| Tegelberg et al | 74 | 55 | A: MAD | 50% of maximum protrusion mean, 4.5 ± 0.93 mm |
Not reported | Questionnaire: | MAD A | Not reported | Not reported | Not reported | Daytime sleepiness decrease |
| (1 piece) | Daytime sleepiness, apneas, and snoring |
MAD B | Not reported | Not reported | Not reported | MAD A: 45 (82%) | |||||
| MAD B: 46 (84%) | |||||||||||
| B: MAD (1 piece) |
75% of maximum protrusion mean, 6.4 ± 1.16 mm |
Not reported | Decrease in apneas and snoring | ||||||||
| MAD A: 48 (87%) | |||||||||||
| MAD B: 43 (79%) | |||||||||||
| Vanderveken et al | 38 | 35 | A: MAD (custom-made monobloc) | 65% ± 10% of maximum protrusion |
Not reported | VAS snoring | MAD A | 8 ± 2 | 2 ± 3 | P <0.01 | Satisfactory reduction in snoring MAD A: 23 (80%) MAD B: 18 (51%) |
| MAD B | 8 ± 2 | 4 ± 3 | P <0.01 | ||||||||
| B: MAD (thermoplastic Somnoguard Plus monobloc) | 50% ± 20% of maximum protrusion |
Not reported | ESS | MAD A | 7 ± 5 | 5 ± 4 | NS | ||||
| MAD B | 7 ± 5 | 6 ± 4 | NS | ||||||||
| Walker-Engström et al | 86 | 77 | A: MAD | A: | ESS | MAD A | 11.7 ± 3.1 | 8.6 ± 2.8 | P <0.001 | ||
| (1 piece) | 50% of maximum | 2 mm | MAD B | 11.5 ± 3.1 | 7.5 ± 2.6 | P <0.001 | |||||
| protrusion | |||||||||||
| mean, 5 mm | |||||||||||
| (4.8-5.3) | |||||||||||
| B: MAD | B: | ||||||||||
| (1 piece) | 75% of maximum | 2 mm | Questionnaire: | MAD A vs MAD B: NS | Satisfaction with MAD A: | ||||||
| protrusion | Daytime sleepiness, apneas and snoring | 69 (90%) patients at 6-mo follow-up were satisfied or very satisfied; 7 (9%) neither satisfied nor dissatisfied Large decrease in daytime sleepiness: MAD A: 33 (43%) MAD B: 42 (54%) Decrease in apneas and snoring: MAD A: 48 (62%) MAD B: 59 (77%) |
|||||||||
| mean, 7.2 mm | |||||||||||
| (6.7-7.6) | |||||||||||
| Author | Patients (n) | Oral appliance (OA) | Advancement | Assessment tool used | Result | Statistical significance | Subjective treatment satisfaction | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Complete | Sagittal | Vertical | Pretreatment | Postreatment | ||||||
| Gauthier et al | 23 | 16 | A: MAD (commercial two-piece Silencer) | 50% of max protrusion average 10.5 mm | 9-12 mm | ESS | MAD A | 13.9 ± 1.3 | 9.9 ± 1.3 | P ≤0.01 | Subjects and sleep partners reported that both MADs significantly reduced snoring frequency, choking, cessation of breathing, number of arousals, daytime sleepiness, frequency of morning headaches, daytime aggressive or irritable reactions and decreased libido ( P <0.05 to 0.001). |
| MAD B | 13.9 ± 1.3 | 9.3 ± 1.2 | P ≤0.001 | ||||||||
| MAD vs MAD: NS | |||||||||||
| B: MAD (commercial two-piece Klearway) | 66% of max protrusion average 12.5 mm | 9-12 mm | FOSQ | MAD A | 13.8 ± 0.7 | 16.8 ± 0.6 | P ≤0.001 | ||||
| MAD B | 17.2 ± 0.5 | P ≤0.001 | |||||||||
| MAD vs MAD: NS | |||||||||||
| VAS | MAD A | Not reported | 6.5 ± 0.5 | MAD vs MAD: NS | |||||||
| MAD B | Not reported | 7.4 ± 0.4 | |||||||||
| FSS | MAD A | 45.4 ± 2.7 | 39.0 ± 2.6 | NS | Cessation of breathing and perception of choking showed greater reduction with Silencer than Klearway ( P <0.05). | ||||||
| MAD B | 39.4 ± 3.6 | NS | |||||||||
| Lawton et al | 16 | 16 | A: MAD (twinbloc) | Not reported | Not reported | ESS | MAD A | 10 (2-18) ∗ | 8.5 (3-17) ∗ | MAD A vs MAD B: NS | Not reported |
| MAD B | 10 (2-18) ∗ | 8.0 (4-18) ∗ | |||||||||
| B: MAD (Herbst) | Not reported | Not reported | SF-36 | All categories ns | |||||||
| VAS: daytime sleepiness | MAD A | 3 (1-4) ∗ | 2.5 (1-4) ∗ | MAD A vs MAD B: P = 0.04 | MAD B less sleepy than MAD A | ||||||
| MAD B | 3 (1-4) ∗ | 2.0 (1-4) ∗ | |||||||||
| VAS: snoring | MAD A | 4.0 (3.0-4.0) ∗ | 4.0 (2.0-4.0) ∗ | MAD A vs MAD B: NS | |||||||
| MAD B | 4.0 (3.0-4.0) ∗ | 3.5 (1.0-4.0) ∗ | |||||||||
| Pitsis et al | 24 | 23 | A: MAD (two-piece) | 87 ± 4% of max protrusion mean 7.3 ± 0.5 mm | 4 mm | ESS | MAD A | 18 ± 1 | 12 ± 1 | P <0.001 | Not reported |
| MAD B | 18 ± 1 | 12 ± 1 | P <0.001 | ||||||||
| B: MAD (two-piece) | 87 ± 4% of max protrusion mean 7.3 ± 0.5 mm | 14 mm | |||||||||
| Patients (n) | Oral appliance | Advancement | Assessment tool | Result | Statistical | Subjective treatment satisfaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Baseline | Complete | (OA) | Sagittal | Vertical | used | Pretreatment | Posttreatment | significance | ||
| Bloch et al | 24 | 24 | A: MAD (monobloc) | 75% of maximum protrusion mean, 10 ± 0.4 mm | 5-10 mm | ESS | MAD A | 9.0 ∗ | (6.5-10) ∗ | MAD A vs no treatment: P <0.001 | Not reported |
| MAD B | 9.0 ∗ | (6.5-11) ∗ | MAD B vs no treatment: P <0.001 | ||||||||
| No treatment | 13.5 ∗ | (9.5-16) ∗ | MAD B vs MAD A: NS | ||||||||
| B: MAD (Herbst) | 75% of maximum protrusion mean, 10 ± 0.4 mm | 4-6 mm range of opening, >15 mm | |||||||||
| Sleep symptoms questionnaire: | |||||||||||
| Interference with daily tasks | MAD A | 1.5 ∗ | (1.0-2.0) ∗ | MAD A vs no treatment: P <0.001 | |||||||
| MAD B | 2.0 ∗ | (1.0-3.0) ∗ | MAD B vs no treatment: P <0.03 | ||||||||
| No treatment | 3.5 ∗ | (2.5-4.0) ∗ | MAD B vs MAD A: P <0.05 | ||||||||
| C: no treatment | – | – | Perform ability | MAD A | 2.0 ∗ | (1.0-2.0) ∗ | MAD A vs no treatment: P <0.001 | ||||
| MAD B | 2.0 ∗ | (2.0-3.5) ∗ | MAD B vs no treatment: P <0.03 | ||||||||
| No treatment | 3.0 ∗ | (2.0-4.0) ∗ | MAD B vs MAD A: P <0.05 | ||||||||
| Energy level | MAD A | 2.0 ∗ | (2.0-3.0) ∗ | MAD A vs no treatment: P <0.001 | |||||||
| MAD B | 2.0 ∗ | (2.0-3.0) ∗ | MAD B vs no treatment: P <0.03 | ||||||||
| No treatment | 3.0 ∗ | (2.5-4.0) ∗ | MAD A vs MAD B: NS | ||||||||
| Snoring frequency | MAD A | 2.0 ∗ | (1.0-3.0) ∗ | MAD A vs no treatment: P <0.001 | |||||||
| MAD B | 2.0 ∗ | (1.0-3.5) ∗ | MAD B vs no treatment: P <0.001 | ||||||||
| No treatment | 4.0 ∗ | (4.0-4.0) ∗ | MAD A vs MAD B: NS | ||||||||
| Snoring loudness | MAD A | 1.5 ∗ | (1.0-2.0) ∗ | MAD A vs no treatment: P <0.001 | |||||||
| MAD B | 2.0 ∗ | (1.0-2.5) ∗ | MAD B vs no treatment: P <0.001 | ||||||||
| No treatment | 3.5 ∗ | 3.5 (3.0-4.0) ∗ | MAD A vs MAD B: P <0.05 | ||||||||
| Rose et al | 26 | 16 | A: MAD (2-piece soft polyethylene Silencor) | 75% maximum protrusion |
5 mm | VAS: daytime sleepiness, |
MAD A MAD B |
7.2 (1.7) 7.0 (1.5) |
5.4 (1.0) 4.1 (0.7) |
Both MADs reduced daytime sleepiness and snoring significantly while | Not reported |
| B: MAD (acrylic 1-piece Karwetzky) | 75% maximum protrusion | 10-12 mm | Snoring Sleep quality |
MAD A MAD B MAD A MAD B |
9.1 (0.8) 8.8 (1.0) 6.4 (1.8) 6.2 (1.2) |
3.2 (1.4) 3.4 (2.7) 4.1 (1.4) 4.5 (2.1) |
Enhancing sleep quality (no details given); no differences were found in this respect between the 2 MADs |
||||
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


