Fig. 4.1
(a–d) The expression 2.3-GFP, Dspp, and DMP–1 in the developing maxillary first molars. Bright field (a), epifluorescence (b) pseudo-colored bright field (c, d) images of the frontal sections through first maxillary molars from postnatal day 4. (a) During the secretory stage of crown development, there is a gradient of odontoblast differentiation in the developing cusp of the first maxillary molars. The terminally differentiated odontoblasts (O) associated with predentin and dentin opposed by ameloblasts (A) secreting enamel are present at the tip of the cusps. Functional odontoblasts (FO) secreting only predentin, pre-odontoblasts (PO), and polarizing odontoblasts (PoO) are present at the cervical loops. (b) 2.3-GFP is not expressed in pre-odontoblasts (PO) or polarizing odontoblasts (PoO), but is expressed at high levels in the functional odontoblasts (FO), in terminally differentiated odontoblasts, and in osteocytes of the developing alveolar bone. (c) Dspp is expressed at high levels by functional odontoblasts (FO) secreting predentin and terminally differentiated odontoblasts (O) associated with predentin and dentin. Dspp is also expressed by a group of early secretory ameloblasts (ESA) located at the cervical loops opposing the functional odontoblasts (FO) expressing Dspp. Pre-odontoblasts (PO), polarizing odontoblasts (PoO), or the osteoblasts of the developing bone (B) do not express Dspp. (d) Unlike Dspp, DMP–1 is expressed at low levels only by functional odontoblasts (FO) located at the cervical loop and not by terminally differentiated odontoblast (O) at the tips of the cusps. DMP–1 is not expressed by pre-odontoblasts (PO) and polarizing odontoblasts (PoO). Also note the high levels of DMP–1 expression by the osteoblasts of the developing bone (B). Scale bar = 200 um
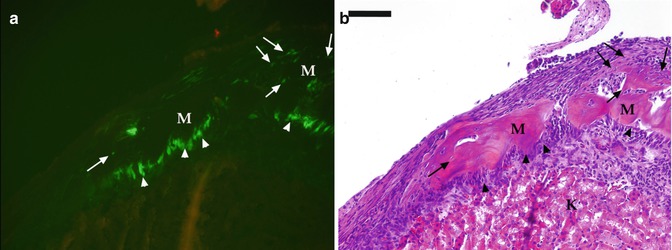
Fig. 4.2
(a, b) Analysis of pulp from pOBCol2.3GFP transgenic mice after grafting under the kidney capsule. Epifluorescent (a) and bright field (b) images of a piece of the coronal portions of pulp isolated from mandibular first molars of pOBCol2.3GFP transgenic mice transplanted for 10 days under the kidney capsule of CD-1 hosts. (a) The formation of odontoblast-like cells (indicated by arrowheads) expressing high levels of 2.3-GFP that are lining the surface of the mineralized matrix (M). Also note the formation of osteocyte-like cells (indicated by arrows) embedded within the mineralized matrix. Scale bar = 200 um
By multiplexing the promoter-GFP colors in which the donor and recipient carry a different color, GFP driven by the same promoter is particularly useful in interpreting a transplantation experiment in that it allows distinction in the contributions of donor vs. host cells.
We also analyzed the temporal and spatial expression of these transgenes during in vitro mineralization and odontoblast differentiation in primary cultures derived from the coronal portions of dental pulp of molars from these transgenic animals. Our studies showed the expression of these transgenes in scattered cells at day 7 before initiation of mineralization and expression of Dspp indicating that, during odontoblasts differentiation, 2.3-GFP and 3.6-GFP are activated at early stages of differentiation [23].
With the ability of FACS to separate cells based on GFP expression, we obtained relatively homogenous subpopulations of Col1a1-GFP + cells and analyzed their proliferation and dentinogenic potentials. Our results showed that 2.3-GFP + and 3.6-GFP + populations contain highly proliferative cells enriched in progenitors committed to mineralization and dentinogenesis shown by sheets of mineralized tissue expressing Dspp in these cultures. Most interesting was the differences in levels of Dspp in different populations. These studies showed these transgenes are activated before the onset of matrix deposition and in cells at intermediate stages of odontoblast differentiation. The 3.6-GFP transgene was activated in cells in early stages of polarization, whereas the 2.3-GFP transgene was activated at a later stage of polarization just before or at the time of transition into secretory odontoblast. These differences were not appreciated in the developing teeth in vivo because of the close proximity of cells in the early and late stage of polarization. Further experiments are in progress to examine the expression of these two transgene in dual GFP reporter mice in which different color GFPs (topaz and cyan) are driven by 3.6 and 2.3 kb of the Col1a1 promoter, respectively. See Fig. 4.3a, b.
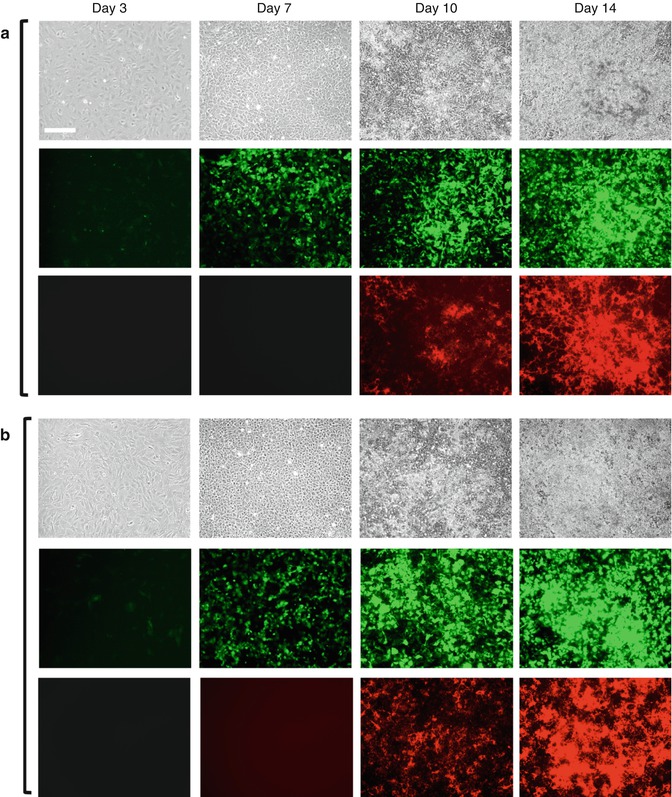

Fig. 4.3
(a, b) Expression of 3.6-GFP and 2.3-GFP transgenes in primary dental pulp cultures during in vitro mineralization and dentinogenesis. Primary dental pulp cultures obtained from pOBCol3.6GFP (a) and pOBCol2.3GFP (b) transgenic animals grown for 14 days in culture conditions supporting their mineralization and dentinogenesis. Images of the same areas in cultures at different time points analyzed under phase contrast (upper rows in a and b), epifluorescent light using filters for GFPtpz and GFPemd for detection of GFP (middle rows in a and b), and epifluorescent light using TRITC red filter for detection of xylenol orange (XO) staining in mineralized matrix (lower rows in a and b). Note the presence of GFP + cells early in the culture (day 3), in cell clusters at day 7, and in differentiating and differentiated nodules between days 10 and 14. Scale bars = 200 μm
We also extended these studies to identify markers for later stages of odontoblast differentiation. Given the important roles of DMP1 in dentinogenesis, we used DMP1-GFP transgenic mice in which GFPtpz is under the control of 8 kb upstream regulatory sequences of DMP-1 [25]. The in vivo studies showed that DMP1-GFP was first expressed at E19 in the secretory/functional odontoblasts prior to the expression of Dspp. The expression of DMP1-GFP intensified in odontoblasts synthesizing primary dentin and was decreased in highly differentiated odontoblasts synthesizing secondary dentin.
Our in vitro studies using pulp cultures from DMP1-GFP transgenic animal showed that during mineralization of primary pulp cultures, DMP1-GFP is expressed in cells in odontogenic and osteogenic lineage. In the osteogenic areas of cultures (identified by the lack of DSP expression), DMP1-GFP was expressed at high intensity. On the other hand, in the dentinogenic areas (identified by the expression of DSP), DMP1-GFP was expressed at two different intensities. The expression of DMP1-GFP at high intensity was associated with secretory/functional and newly differentiated odontoblasts, whereas the expression at very low intensity was associated with highly differentiated odontoblasts [25].
Our in vitro studies also showed that the behavior of DMP1-GFP + population was different from 2.3-GFP + populations. Unlike the 2.3-GFP + population, DMP1-GFP + cells exhibited poor proliferation and survival, suggesting that this population contains postmitotic cells in advanced stages of differentiation (Fig. 4.4). The lack of detectable levels of Dspp in DMP1-GFP + population isolated at day 7 (early time point) from pulp cultures suggested that DMP1-GFP is activated in secretory odontoblasts prior to the expression of Dspp [25].
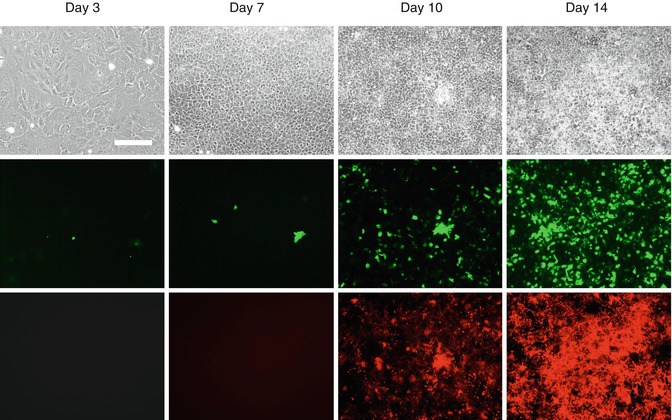

Fig. 4.4
Expression of DMP1-GFP transgenes in primary dental pulp cultures during in vitro mineralization and dentinogenesis. Primary dental pulp cultures obtained from DMP1-GFP transgenic animal grown for 14 days in culture conditions supporting their mineralization and dentinogenesis. Images of the same areas in cultures at different time points analyzed under phase contrast (upper row), epifluorescent light using filters for GFPtpz and GFPemd for detection of GFP (middle row), and epifluorescent light using TRITC red filter for detection of xylenol orange (XO) staining in mineralized matrix (lower rows). Scale bars = 200 μm
It is important to note that the 2.3-GFP, 3.6-GFP, and DMP1-GFP transgenes, similar to expression of the endogenous proteins, are expressed by both odontoblasts and osteoblasts and therefore make it difficult to distinguish between the two cell types. To overcome this difficulty, we have generated Dspp-Cerulean transgenic mice that show restricted expression of transgene in odontoblasts.
These studies together showed the heterogeneity of pulp cultures and the necessity for development of markers for identification and isolation of more homogenous population for careful lineage analysis. In these cultures the 3.6-GFP transgene was activated in cells in early stages of polarization, whereas the 2.3-GFP transgene was activated at a later stage of polarization just before or at the time of formation of secretory/functional odontoblast. This is followed by the activation of DMP1-GFP transgene in secretory/functional odontoblasts (Fig. 4.5). These studies also indicate that these transgene can be used for identification and isolation of cells at different stages of odontoblast differentiation from the heterogeneous population of dental pulp cells. The ability to isolate relatively homogenous populations using GFP strategy during odontoblast differentiation by FACS sorting has the potential to overcome shortcoming of microarray analysis of whole cultures and should provide new expression profiles of stage-specific genes during normal and abnormal dentinogenesis in mice with various genetic mutations.
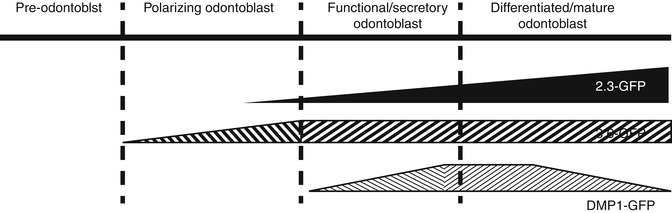

Fig. 4.5
Schematic representation of proposed stages of activation of 3.6-GFP and 2.3-GFP transgenes during odontoblast differentiation. DSPP was used as a marker of early and later stages of mineralization
4.3 Dental Pulp Progenitor and Stem Cells
Dentin secreted by odontoblasts during tooth development and before the completion of root formation is defined as primary dentin and is deposited at a rate of 4–20 μm/day. Following primary dentinogenesis, secondary dentin is secreted throughout life at a much slower rate (0.4 μm/day) and results in a decrease in the size of the pulp chamber. Primary and secondary dentins secreted by odontoblasts are characterized by closely packed dentinal tubules that span the entire thickness of the dentin. However, the function of odontoblasts is not limited to primary and secondary dentinogenesis, but includes maintenance of dentin thorough the life of the tooth.
The dentin–pulp complex has a regenerative potential leading to the formation of tertiary dentin. In response to mild environmental stimuli (attrition or early caries), preexisting live odontoblasts upregulate their secretory activity and secrete a tubular reactionary dentin matrix [29, 30]. This layer shows many anatomical, biochemical, and functional similarities to the primary and secondary dentins and contributes to the protection of the pulp tissue. Reactionary dentin formation is promoted by low amounts of pro-inflammatory cytokines and/or growth factors and extracellular matrix components sequestered in dentin that are released following dentin demineralization by cariogenic bacteria.
On the other hand, trauma of greater intensity that causes the death of the preexisting odontoblasts leads to reparative dentinogenesis involving the recruitment and proliferation of progenitor and/or stem cells to the site of injury and their differentiation into a second generation of odontoblasts or “odontoblast-like cells” [29, 30]. These odontoblast-like cells synthesize an atubular dentin-like mineralized matrix immediately below the site of damage to preserve pulp vitality [29, 30]. The reparative dentin also referred to as osteodentin contains cells trapped within the matrix that forms in the absence of inner dental epithelium and basement membrane. Reparative dentinogenesis is thought to be dependent on multiple signaling molecules sequestrated in the dentin matrix [29, 30].
Potential populations of cells within dental pulp capable of giving rise to the new generation of odontoblast-like cells during reparative dentinogenesis are many and include the cell-rich layer of Höhl adjacent to the odontoblasts, fibroblasts, and progenitors/mesenchymal stem cells (MSCs) [29, 30]. Available evidence suggests that the dental pulp contains several niches of potential progenitors and stem cells involved in reparative dentinogenesis.
The discovery of stem cells from human dental pulp of impacted third molars in the year 2000 (DSTP) and later in the pulp of exfoliated primary teeth (SHED) suggested that these populations are among the potential populations involved in reparative dentinogenesis [31, 32]. Transplantation of in vitro expanded DPSCs and SHED mixed with hydroxyapatite/tricalcium phosphate particles into immunocompromised mice resulted in the formation of pulp–dentin-like tissue complexes including vascularized pulp-like tissue, surrounded by a layer of odontoblast-like cells without an active hematopoietic marrow [31, 32]. Transplantation of SHED seeded in tooth slice/scaffolds into the subcutaneous space of immunodeficient mice also resulted in the generation of tissue-like human dental pulps [33]. The odontoblast-like cells in these experiments expressed dentin sialoprotein (DSP), a marker for odontoblastic differentiation [31–33].
A recent study provided strong evidence for the differentiation of SHED into functional odontoblasts by demonstrating that these cells were able to generate new tubular dentin in vivo, as determined by tetracycline staining [34]. These studies suggested that human adult dental pulp contained a small population of self-renewing, highly proliferative multipotent mesenchymal stem cells (MSCs) that reside within a larger population of more committed progenitors [35]. These cells exhibit similar characteristic with bone marrow stromal stem cells (BMSCs), including fibroblast-like morphology, adherent colony-forming units.
MSCs, first described in postnatal bone marrow [36], constitute a rare population of cells of non-hematopoietic origin that were identified by their adherence to plastic tissue culture dish and their fibroblastic morphology. When transplanted into an animal, MSC from bone marrow form bone, cartilage, hematopoietic marrow, fat cells, and the stroma that supports blood formation [37]. On the basis of their origin and their multipotency, these cells were originally referred to as “bone marrow stromal stem cells” (BMSCs) and more recently MSC [37].
Since their discovery in the bone marrow, MSCs have been identified in almost every organ in the body using criteria established for BMSCs including dental pulps. Furthermore, different populations of MSCs have been isolated from dental pulp of various teeth in humans and animal models.
All of these populations are heterogeneous in that cell colonies derived from these cells exhibit morphological and functional (cell proliferation and differentiation) similarities and differences. These cells are all capable of regenerating dentin and pulp-like tissues after transplantation [35]. The fraction of multipotent stem cells in the dental pulp is small as defined by their in vitro ability to undergo odontogenic, angiogenic, adipogenic, chondrogenic, neurogenic, or myogenic differentiation.
Comparative studies demonstrated that MSC isolated from different organs, including dental pulps, exhibit many differences including differences in the expression of stem cell markers, proliferation, and osteogenic differentiation [35]. Furthermore, there are significant differences in their multipotency and expression of markers of pluripotency and in their differentiation potential that is related to the developmental stage at which a stem cell is isolated.
Although these studies have provided evidence that the cells are able to form the dentin pulp complex, the origin/identity of the progenitor cells and signaling pathways involved in reparative dentinogenesis in vivo remain elusive. This is, in part, due to the lack of suitable markers for the identification of potential progenitors and appropriate animal models to trace the fate of potential progenitors during reparative dentinogenesis.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


