Fig. 12.1
Slight reactionary dentin formation with no persistent inflammation after atraumatic cavity preparation and application of a nontoxic material in a medium deep cavity (magnification ×80) (Used with permission of Springer Science+Business Media from Schmalz and Arenholt-Bindslev [2])
Non-rotary methods for cavity preparation may comprise alumina air abrasion, lasers, or oscillating instruments. For air abrasion no heat generation was reported [9]. Er:YAG laser-based preparation tools seem to produce less temperature increase than ruby, CO2, and Nd:YAG lasers, the later above pulpal tolerance when used on mineralized tissues [5, 10]. For oscillating instruments again water coolant is necessary, but 7.3 ml water/min was reported to be sufficient [11] and the relevant ISO Standard 18397:2013 requires a minimum of 20 ml/min cooling liquid.
12.3 Symptoms
Generally, clinical symptoms like pain in its different manifestations are a rather unreliable indicator for pulp disease (see Chap. 9) and, therefore, also for material-related pulp damage. The absence of pain is definitively not a sign that no – histologically detectable – pulp damage has occurred. Histological studies in human teeth after pulp capping with dental adhesives clearly showed that the teeth were clinically asymptomatic although histological evaluation proved severe inflammation [12].
If pain occurs after material application, this indeed may be due to a substance diffusing to the pulp. This is quite often – up to 65 % – observed after the application of (highly concentrated) hydrogen peroxide-releasing tooth-whitening products [13], and it may be related to hydrogen peroxide diffusion through the enamel and dentin to the pulp [14]. On the other hand, similar symptoms can be observed after the application of resin-based composite restorations (“postoperative sensitivity”), which are mainly related to the intratubular fluid movement after masticatory load (see the discussion to come).
Besides pain, other symptoms may be the consequence of material tissue interaction like discoloration of the tooth after pulp necrosis. Furthermore, tertiary dentin formation (biomineralization) can be the consequence of material application. Interestingly, dentin formation can also be inhibited, e.g., by pulp capping with resin materials [8, 12, 15] with the clinical consequence of deficient or lacking dentin bridge formation.
Taken together the clinical diagnosis of material-related pulp damage is very complicated, and absence of clinical symptoms is no indicator for pulp biocompatibility. Therefore, all possible and reasonable measures must be undertaken to prevent pulp damage as best as present knowledge allows.
12.4 Reasons
A prime reason for pulp damage is the direct interaction between a (toxic) substance released from a material and a relevant cellular or another molecule within the pulp tissue [16]. Virtually all dental materials release substances especially during and shortly after setting [17, 18], which then contact vital (often freshly cut) dentin and which can diffuse toward the pulp [19, 20] (Fig. 12.2). In vital teeth, dentinal liquor is diffusing from the pulp outward to the material contact area possibly causing (partial) dissolution of the material and an impairment of the setting reaction. This is especially pronounced, if the material is in direct contact with the exposed pulp: due to the high degree of wetness, comparatively large amounts of substances are released from the material. The amount/concentration of the released substance at the target site (pulp cells) depends on a number of variables, an important one being the thickness of the residual dentin (see the discussion to come).
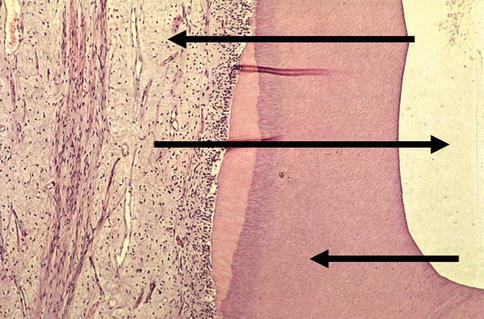

Fig. 12.2
Material-dentin/pulp interaction: release of substances from the applied material which diffuses through the dentin to the pulp (upper arrow), outflow of dentin liquor after cavity preparation (middle arrow), and adsorption of released substance within the dentin (lower arrow) (magnification ×80) (Used with permission of Springer Science+Business Media from Schmalz and Arenholt-Bindslev [2])
Further to this direct material tissue interaction, pulp damage after restorative procedures was also related to bacteria at the cavity floor (indirect interaction). Especially under resin-based composite restorations, bacteria at the cavity floor [21] were consistently found in cases of pulp inflammation both in experimental animals and in humans [22], and thus bacteria were assumed to be the cause of the pulp damage [22] (Fig. 12.3a, b). Bacterial penetration can occur through microgaps at the material/dentin interface. The use of adequate dentinal adhesives markedly reduces the bacterial penetration, but still penetration studies reveal quite some amount of microleakage, more in methacrylate-based materials than with siloran-based products [23, 24]. However, in such studies mainly dyes are used for quantifying microleakage, and it is still unclear if this can be related to bacterial penetration in vivo [25]. On the other hand, a material-related reduced bacterial clearance [26, 27] and the ability of monomers to stimulate bacterial growth [28] could further promote bacterial presence at the cavity floor.
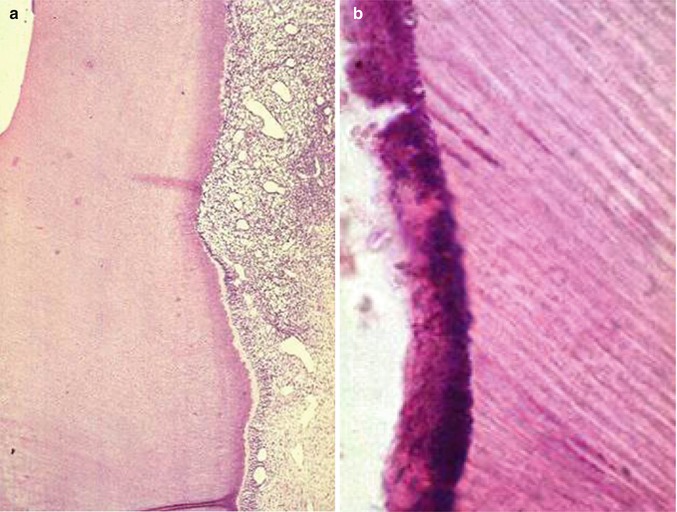

Fig. 12.3
Strong pulp inflammation (a magnification ×80) associated with bacteria at the cavity floor under the restoration (b, magnification ×400) (Used with permission of Springer Science+Business Media from Schmalz and Arenholt-Bindslev [2])
Heat is a point of concern not only for cavity preparation (see earlier discussion) but also for light-curing units, which are needed for resin polymerization. Recently, such devices with a high energy output were marketed, and especially close to the pulp, heat-related pulp damage is possible [29] (see also the discussion to come).
Mechanically caused fluid displacement in the dentinal tubule has been described to be the reason for postoperative sensitivity after placement of resin-based composite restorations [2]. This is mainly observed after occlusal loading, and it has been postulated that due to insufficient bonding and the formation of microgaps between the cavity floor and the restoration, a pump effect occurs after masticatory load, which causes a fluid movement in the dentinal tubule with consecutive pain. Using dentinal adhesives reduces markedly sensitivity [2]; some authors favor in this respect self-etch adhesives [30].
12.5 Residual Dentin
Although dentin contains a large number of tubule, in which odontoblastic processes and lateral processes are located [31], it has been reported that dentin (even if cut) has a protective effect on the pulp especially for substances released from materials. To demonstrate this effect, the influence of different dentin thicknesses/distances from the pulp on different parameters has been studied. In vitro, dentin permeability was shown to be dependent upon dentin thickness [31], and permeability exponentially increased with decreasing dentin thickness especially at <500 μm (Fig. 12.4) [31]. This corresponds to the fact that – according to Hagen-Poiseuille’s law – permeability is inversely related to the length of the tubule (dentin thickness). Furthermore, the number and the diameter of the tubule close to the pulp are considerably larger than at the dentin-enamel junction [31] (Fig. 12.5a, b), by which permeability is further increased close to the pulp. Therefore, the residual dentin acts as a barrier reducing the amount of substances reaching the target cells (pulp cells) [31] being especially effective for residual dentin thicknesses of >500 μm. In accordance with that cell culture studies showed that dentin thickness between test material and target cells can reduce cell toxicity of test materials [15]. The same is known from in vivo studies on animals and on humans [2].
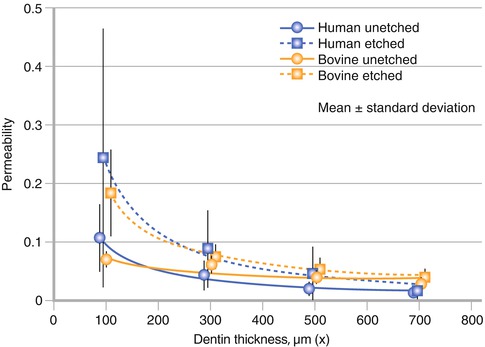
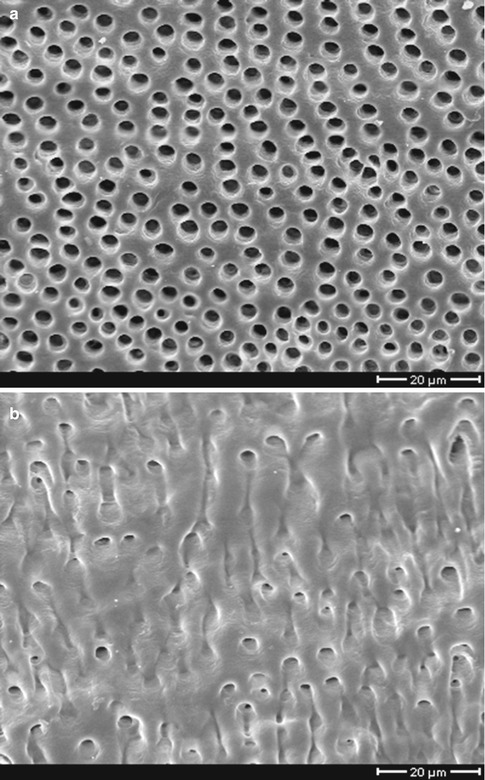

Fig. 12.4
Permeability of dentin at different dentin thicknesses with and without etching (Used with permission of Schmalz et al. [31])

Fig. 12.5
Anatomy of dentin (a) close to the pulp more tubules with larger diameter than (b) close to the dentin-enamel junction (Used with permission of Springer Science+Business Media from Schmalz and Arenholt-Bindslev [2])
This effect is modified by several factors: the first being dentinal sclerosis. Mineral deposition within the dentinal tubule occurs as a result of long-term irritation, e.g., by caries. Dentin permeability depends – according to the aforementioned Hagen-Poiseuille’s law – to the fourth power on the tubule diameter. Therefore, a reduction of the tubule diameter, sometimes even the total closure of the tubule, decreases considerably dentin permeability and thus the diffusion of possibly toxic molecules toward the pulp.
A second modifying factor is the so-called smear layer, which is produced after grinding dentin, e.g., during caries removal and cavity preparation. Removal of the smear layer increases dentin permeability [32]. However, we found that the removal of the smear layer, e.g., by an acid does effect dentin permeability mainly in thin dentin slices [31], i.e., in deep cavities (<0.5 mm residual dentin) (Fig. 12.4). This may be an explanation of the fact that in median and shallow cavities, acid treatment of dentin does apparently not cause pulp damage. A final modifying factor to be considered is the adsorption of some substances released from materials like Zn (from amalgam) or eugenol (from respective cements) to the inorganic dentin component (hydroxyl apatite) of dentin or to proteins [43, 46].
All these factors influence the concentrations of substances released from the material, which reach the dental pulp with the consequence that material-related pulp reactions are mainly expected in deep cavities (i.e., <0.5 mm residual dentin thickness) and after pulp exposure. This means that dentin indeed has a protective effect in these cases.
Interestingly, pulp reactions toward resin-based materials were observed in medium cavities, if bacteria were present (see earlier discussion). Apparently, the protective effect of dentin, which is observed after material application, is not operating against bacteria. An explanation may be that the concentration of substances released from materials decreases with time [17], but the amount of toxicants produced by bacteria on the cavity floor may increase over time by bacterial growth, if, e.g., through gaps, bacteria receive sufficient nutrition from the oral environment. Apparently, the protective capacity of dentin is not high enough to cope with this bacterial challenge.
12.6 Pulp Biocompatibility Testing
Studies on pulp biocompatibility of dental materials are done (1) to elucidate pulp reactions and the mechanisms behind them and (2) in the course of legally required premarket safety testing. Dental restorative materials are – meanwhile worldwide – regarded as medical devices and legally regulated. Generally, the manufacturer is responsible for the safety of his products, and the manufacturer defines the indication for each product. The dentist is responsible for keeping to the given indication and for the correct processing of the materials.
Medical devices must fulfill certain requirements before they are allowed to be marketed. These requirements also cover safety aspects including pulp biocompatibility. For this, sets of test methods are compiled in ISO standards (ISO 10 993-series, ISO 7405) [2]. In an attempt to fulfill the legal requirements, manufacturers must complete a clinical risk assessment according to ISO 14971, mainly based on these ISO standards [2]. Basically, these tests comprise elution tests (chemical determination of substances released from a material into a liquid) and cell culture tests (for cytotoxicity and for mutagenicity). If necessary, animal studies and studies on humans (e.g., teeth to be extracted for orthodontic reasons) are possible [2]. Today, for assessing the risk of pulp damage, mainly cell culture-based studies are performed, because (large) animal studies are expensive, time consuming, and not without ethical problems. In special cases (completely new chemistry or new materials claiming pulp repair/regeneration), they may become necessary; in cases of only “improved” materials, they can possibly be waived, if cell culture tests give no indication of a possible toxic behavior [2]. The same is true today for long-term animal-based toxicity/carcinogenicity studies.
Classical cell culture methods are fast and comparatively easy to perform, but they do not reflect the clinical situation of a tooth cavity. Therefore, the extrapolation of the obtained data to the patient is problematic, and data must be carefully interpreted. In order to bridge the gap between the classical in vitro cell culture cytotoxicity tests and the patient situation, the so-called dentin-barrier test has been developed, in which a dentin disk of a defined thickness is placed between the material and the target cells [33, 34]. This test method is also accepted as ISO standard (ISO 7405) [35]. Other models mainly being used to elucidate reaction mechanisms are the tooth slice model from human [36] or rat [37] teeth or the whole tooth model [38].
Pulp studies on small laboratory animals, on large animals (e.g., nonhuman primates), or even on human teeth are performed under ideal conditions and thus are not without limitations; e.g., sound teeth of young animals or patients are used for testing, which is in contrast to most patients situations presenting teeth with a caries-affected dentin and pulp. Therefore, preclinical assessment of pulp biocompatibility of dental materials is a first and necessary step, which must be based on a battery of different tests. Even then one cannot assume that all possible side effects can be foreseen by such an evaluation. Therefore, according to legal regulations it is compulsory for the dentist to report observed adverse effects to relevant authorities in the respective country (postmarket surveillance).
12.7 Reaction Patterns
Pulp reactions toward materials/irritants are complex events, and their outcome is determined by the:
-
Properties of the material/irritant (e.g., toxicity and change of toxicity over time, diffusibility in an aqueous system)
-
Local cavity situation (e.g., the amount and the quality of residual dentin)
-
Healing capacity of the pulp (e.g., age, functioning immune system or preexisting caries-induced inflammation)
In the following, pulp reactions in three scenarios (slight irritation, strong irritation, pulp exposure) are described, however, keeping in mind that in reality combinations of these reactions occur.
Slight irritations (gentle cavity preparation in medium cavities filled with materials of only initially medium but then low toxicity or with low diffusibility due to high hydrophobicity and in the absence of bacteria) will be associated with a temporary (few days) inflammatory pulp reaction. If there is no further irritation, the inflammation will resolve, and new dentin (“reactionary dentin”) will be formed by survived odontoblasts at the end of the cut dentinal tubule [39] (see also Fig. 12.1). Reactionary dentin is delineated from the preexisting dentin by a calcio-traumatic line [8]. This new dentin (also called tertiary dentin) is more irregular in its structure than primary and secondary dentin, but normally dentin tubule can be clearly seen and tubular continuity with secondary dentin may exist [39]. Apparently, the odontoblasts can repair their cut processes, if injury does not happen close to the cell body [8]. Further to the new dentin formation, apposition of peritubular dentin and precipitation of intratubular crystals occur, which reduce dentin permeability and serve as a further protective shield [39].
Strong and/or persistent irritations lead to a more extended pulpal inflammation, e.g., in cases of bacteria at the cavity floor under the restorative material. The inflammation will not resolve and potentially lead to pulp necrosis (see also Fig. 12.3). Normally, odontoblasts do not survive under these conditions, and the recruitment of new (secondary) odontoblasts (see the discussion to come) will be hampered by the extensive inflammation process.
Pulp exposure is always associated with the loss of the original (primary) odontoblasts, and new odontoblasts cannot regenerate from neighboring odontoblasts by cell division, because odontoblasts are terminally differentiated postmitotic cells [8]. Such tissue damage always leads to a pulp inflammation. If the irritation is timely restricted and after an appropriate treatment (e.g., by application of a calcium hydroxide-releasing preparation), it is apparently possible that new odontoblasts are recruited from pulpal (stem/progenitor) cells. Such cells are located in different areas of the pulp and in the periapical area, often in perivascular niches. These cells now proliferate, migrate to the pulp exposure site, and differentiate into secondary odontoblasts, which then form new dentin (reparative dentin, dentinal bridge formation). This new dentin may be rather irregular and atubular (fibrodentin/osteodentin) and may contain voids and tunnels especially close to the material tissue contact area. However, it is a new hard tissue with the potential to protect the dental pulp from further injury, if treated properly [8] and bacterial invasion is avoided, e.g. by a restoration tighly sealing the cavitiy walls against bacterial penetration.
The mechanisms behind these different pulp responses toward different irritation scenarios are not fully understood (for more details, see, e.g., [8, 39]). Apparently, odontoblasts constitute the first line of defense, and their reaction determines the further consequences reaching from transient inflammation to necrosis. They are assisted by antigen-presenting dendritic cells, of which one population is present in the odontoblastic region and the other dendritic cell population is located in the central part of the pulp. These cells form a continuous reticular network involving the entire pulp tissue, including the odontoblastic layer [40, 41, 60]. They allow a delayed-type hypersensitivity reaction toward relevant materials [40]. The inflammation itself is characterized by an invasion of cells like neutrophils, lymphocytes, or macrophages. In this context it is interesting that dental monomers reduce the antibacterial defense mechanism of macrophages; these cells normally produce inflammatory mediators like interleukin-6 after a bacterial (LPS) challenge with the aim to eliminate the bacteria through an inflammatory process. Dental monomers impair this synthesis and this may interfere with bacterial clearance [26, 27].
For pulp repair a transient inflammation is apparently necessary [39]. Furthermore, for the formation of new dentin, bioactive molecules are involved like members of the TGF-β family, including TGF-β1, TGF-β3, and BMP-7 [4]. These molecules are available, e.g., from residual dentin after injury and after solubilization by substances like calcium hydroxide or EDTA [1] (Fig. 12.6). Material cell interaction may lead to cell necrosis with a consecutive propagation of inflammation, which then may interfere with pulp repair, especially when the pulp is exposed [3]. An existing bacterial-induced inflammation (due to a caries process) impairs pulp repair [41] and thus influences the pulp reaction to dental materials.
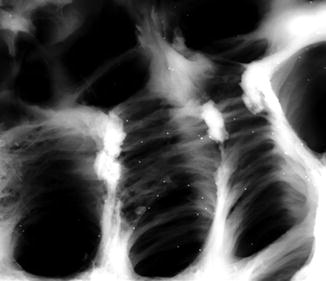

Fig. 12.6
Gold labeling of exposed TGF-β1 (white dots) from dentin (after EDTA treatment)
Also, substances released from materials may induce apoptosis. For instance, dental monomers increase intracellular production of reactive oxygen species (ROS) with consecutive DNA damage [16]. Cells may be able to repair the DNA damage or they go into apoptosis [42]. Apoptosis is not followed by inflammation, but repair and apoptosis are highly energy consuming. This may interfere with the ability of cells to differentiate and to induce biomineralization [43].
With increasing age of the patient, the size of the pulp decreases, and the number of odontoblasts, the number of the cells in the subodontoblast layer and, the number of pulp fibroblasts decreases [44]. Due to a reduced blood flow, removal of toxicants is reduced [39]. The reduced number of odontoblasts together with a reduced secretory activity suggests a compromised repair capacity for reactionary dentin formation for elderly persons. The same is true for the reduced number of fibroblasts in cases of pulp exposure. Therefore, with increasing age the repair capacity of the pulp in general decreases [44].
In summary, the final pulp response to a material is dependent not only upon the material itself but also on the local situation and the status of the pulp. Concerning the material itself, not only biologic interactions are important but also the sealing ability of a material to prevent bacterial migration under the restoration. And finally, concerning the biologic properties of materials, they should be not only nontoxic but also antibacterial and repair stimulating. A problem is that antibacterial materials are very often toxic to (pulp) cells, because the mechanism of the antibacterial effect of dental materials is mainly unspecific and of a multitarget nature. Solutions of this problem can be (1) that materials are only initially antibacterial/toxic eliminating bacteria, but after setting they are inert, allowing for healing and repair, and that (2) toxic materials with high hydrophobicity (like eugenol) are toxic against bacteria at the cavity floor, but due to the low diffusion through the hydrophilic dentin, the effective concentration in the pulp is much lower, and thus it is nontoxic to pulp cells [45].
12.8 Dental Materials/Substances
12.8.1 Amalgam
Amalgam is cytotoxic when freshly mixed but toxicity is significantly reduced after setting [46]. The same was shown in rat subcutaneous implantation studies [47]. Initial local toxicity of zinc-containing and high copper amalgam was higher than zinc-free or low copper products [2, 48]. Eluted metals are bound to the dentin of the cavity floor and are responsible for dentin discoloration. Small amounts of metals are penetrating to the dental pulp. In median and shallow cavities (>0.5 mm residual dentin), usually only slight or no inflammatory reactions are observed several months after application. An apposition of tertiary dentin may be the only histological indication of a pulpal effect [2].
Postoperative sensitivities after placement disappear within a few days. Dentin discoloration can be prevented by using a cavity varnish or a dentinal adhesive. In deep cavities, a cement layer beyond the amalgam restoration is recommended, because due to the amalgam application technique (condensation pressure) the residual dentin may be destroyed and a material placed into the vital pulp. The topic of systemic toxicity of amalgam is not discussed here, but the reader is referred to the literature (e.g., [2]).
12.8.2 Cements
Cements comprise a large group of materials, which normally consist of a powder (zinc oxide or silicon dioxide) and a liquid (often an acid), which both are mixed and which harden by chemical reactions. Silicate cements and silicophosphate cements are virtually not used any more in dental practice. Both material groups were reported to be associated with pulp damage [2].
Zinc phosphate cements are still used, e.g. for luting indirect restorations although it is today mainly replaced by glass ionomer cements. Zinc phosphate cements are briefly after mixing cytotoxic [49] and cause initial pain when used for luting purposes. Cytotoxicity decreases after total setting. In medium and shallow cavities, no long-term pulp damage has been reported, but in deep cavities a protective layer of a calcium hydroxide preparation is recommended [50].
Glass ionomer cements are less cytotoxic than zinc phosphate cements [49]. In shallow, medium, and even deep cavities, no pulp damage was reported, if bacterial penetration to the cavity floor was prevented [50, 51]. However, if glass ionomer cement directly contacted the exposed pulp, severe inflammatory reactions have been observed [51]. Furthermore, in deep cavities, after several weeks no tertiary dentin formation was observed. This can be interpreted as an inhibition of biomineralization. Therefore, again, in deep cavities a protective calcium hydroxide cement layer has been recommended [2, 51].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


