Oral cavity and oropharyngeal cancer account for more than 7000 deaths annually, with an incidence of over 30,000 new cases within the United States. Worldwide it is the sixth most common malignancy with incidence varying greatly among different geographic locations. Its prevalence can range from countries with rates similar to those in the United States, to countries such as India, where death from oral cavity cancer is one of the top three forms of cancer death. Even within the U.S. population, a fivefold difference in mortality is seen among states, likely a result of differences in ethnic and socioeconomic factors as well as the use of tobacco products. Despite improvements in treatment, long-term survival rates are relatively unchanged over the last 30 years. For the oral and maxillofacial surgeon, an understanding of this disease and its impact upon society as well as the surgeon’s role in identification and treatment is imperative.
ANATOMY
The oral cavity extends from the vermilion boarder of the lips to an imaginary coronal plane at the junction of the hard and soft palates and the circumvallate papilla. There are seven anatomic subsites included within the oral cavity: lips, buccal mucosa, lower and upper alveolar ridges, hard palate, floor of mouth, tongue (anterior two thirds), and the retromolar trigone. Subsite variation in cancer incidence, lymph node drainage, and overall prognosis exists.
Lymph node basins that drain the oral cavity are divided into five groups based on anatomically defined structures.
- •
Level I—includes the submental nodes bounded by the anterior bellies of the digastric muscles and the hyoid bone as well as the submandibular group bounded by the body of the mandible and the posterior bellies of the digastric muscles bilaterally.
- •
Level II—includes the upper jugular lymph nodes extending from the base of the skull to the level of the carotid artery bifurcation. The anterior border is the lateral aspect of the sternohyoid muscle with the posterior border of the sternocleidomastoid (SCM) muscle serving as the posterior border of the level. Level II is also subdivided into levels IIA (inferior medial) and IIB (superior lateral) by the spinal accessory nerve.
- •
Level III—includes nodes located adjacent to the middle third of the internal jugular vein from the carotid bifurcation to the omohyoid muscle, with the same anterior and posterior boundaries as level II.
- •
Level IV—includes the lower jugular group, inferior to the omohyoid and superior to the clavicle, with the same anterior and posterior boundaries as levels II and III.
- •
Level V—includes nodes anterior to the anterior border of the trapezius and posterior to the posterior border of the SCM. The inferior border of this level is the clavicle.
BUCCAL MUCOSA
The buccal mucosa includes all mucous membranes lateral to the alveolar sulcus from the lips anteriorly to the retromolar trigone and pterygomandibular raphae posteriorly. Lymphatic drainage from these sites is primarily to levels I and II in the neck. It should be noted that cancer of the buccal mucosa can perforate beyond the buccinator muscle to the buccal fat and subcutaneous tissues, which may drain to the periparotid lymph nodes.
ALVEOLAR RIDGE
The alveolar ridges include the maxillary and mandibular alveolar processes and the overlying mucosa. On the maxilla, this extends from the buccal sulcus to the hard palate. In the mandible, the alveolar ridge extends from the buccal sulcus to the free muscosa of the floor of the mouth. Lymphatic drainage from the upper and lower buccal aspects is to the submandibular and submental (level I) nodes. The lingual aspects of the upper and lower alveolar ridges are to the submandibular (level I), superior deep jugular (level II), and retropharyngeal nodes.
FLOOR OF THE MOUTH
The floor of the mouth extends from the lower alveolar ridge to the ventral surface of the tongue. Its posterior limit is the anterior tonsillar pillar. The floor of mouth has both superficial and deep drainage systems that drain to the submandibular nodes (level I) and to levels I, II, and III, respectively. Crossover drainage to the contralateral side has been demonstrated in anterior portions of both the deep and superficial systems.
RETROMOLAR TRIGONE
The retromolar trigone (RMT) is the triangular region overlying the ascending ramus of the mandible. The base is formed by the last tooth in the arch with the apex at the maxillary tuberosity. Medial and lateral borders are formed by the anterior tonsillar pillar and the buccal mucosa. The mucosa in this region is tightly adherent, leading to early bony invasion. Lymphatic drainage is primarily to the superior deep jugular (level II) nodes with some drainage from this region to the periparotid and retropharyngeal nodes.
HARD PALATE
The hard palate extends from the superior alveolar ridge to the palatine bones posteriorly. Tumors in this region may access additional sites through the incisive and greater palatine foramina. Drainage from the palate is primarily to levels I and II.
TONGUE
The anterior two thirds of the tongue (mobile tongue) anterior to the circumvallate papillae is included in the oral cavity while the base is a part of the oropharynx. Six paired muscles form the mobile tongue. Lymphatic drainage is extensive and has been divided into anterior, lateral, central, and posterior groups. The primary basins include levels I, II, and III. More anterior lymphatic channels drain first to submental nodes, which further drain to lower jugulo-omohyoid nodes. The posterior compartments drain primarily to upper jugulodigastric nodes. As with the floor of the mouth, communication across the midline exists.
EPIDEMIOLOGY
RISK FACTORS
Tobacco
Large, well-designed, population-based studies affirm the correlation between tobacco use and the risk of oral cavity cancer. Tobacco smoking is an independent risk factor; compared to nonsmokers, smokers are six to eight times more likely to develop oral cavity cancer. This is especially true for women, who are twice as likely as men to develop oral cancer given the same amount of tobacco consumption. More than 300 chemicals in tobacco smoke have been identified as contributing to carcinogenesis. It is thought that exposure to these carcinogens leads to malignant transformation of cells. Smoking cessation is effective in reducing risk, though not in its complete elimination. Individuals who refrained from smoking 1 to 9 years showed a 30% reduction of risk, with a 50% reduction for individuals abstaining from smoking more than 9 years.
Outside of cigarettes, both pipe and cigar smoking increase the risk for the development of oral cancer. The role of smokeless tobaccos within the United States has long been accepted to increase cancer risk, especially gingival and buccal cancers. A North Carolina-based study from 1986 showed a four times increase in risk in women users of smokeless tobacco. Recently some have argued the results of this study, claiming a lack of causation. Bouquot and Meckstroth reviewed statistics from West Virginia (the highest per capita consumption of smokeless tobacco) in regard to incidence and mortality of oral and pharyngeal cancer, concluding that rates were less than the national average. Accortt et al categorized 6779 subjects 45 to 75 years of age. In their study, exclusive use of smokeless tobacco was not shown to increase incidence of oral cancer in males or females. No synergistic effects were observed between smoking tobacco and smokeless tobacco. The use of smokeless tobacco, however, has been correlated with other cancer risk factors, with a recommendation for educating these patients regarding their overall cancer risk. It is likely that the debate of causation for smokeless tobacco and oral cancer will continue and patients should be aware at present that risks may exist.
Other forms of chewed tobacco have been shown as independent risk factors for oral cancer. Likely the most prominent is use of tobacco and betel nut in parts of India and Southeast Asia. In one study the incidence of oral cavity cancer was 123-fold higher for individuals who smoke and chew betel nut. These data infer that 40% to 50% of all cases of oral cavity cancer in this geographical region occur in individuals with this habit.
Alcohol
The relationship between alcohol and cancer is clear for hard liquor and high-level alcohol consumers but less well-defined in other risk groups. Approximately 75% of all patients who develop oral cancer do consume alcohol at some level. One Swedish-based study of more than 1300 individuals showed moderate alcohol intake alone (<10 g/day) did not increase risk significantly but high intake (>50 g) was an independent risk factor with a relative risk of 5.5. In addition, alcohol in conjunction with smoking was synergistic, with a relative risk of 22.1 compared to a relative risk of 6.5 from smoking alone. In another study looking at extreme alcohol consumers, the risk for cancer from alcohol has been found to be greater than that from smoking alone. Alcohol may predispose to cancer in any location in the oral cavity and is thought to possibly contribute more highly to dependent sites such as the floor of the mouth and lower buccal vestibule.
Viruses
A number of human viruses have been suggested as etiologic agents for oral cavity cancer through their transformation of human DNA. Herpes simplex virus type 1 (HSV-1) and human papillomaviruses (HPVs) have been reported by various authors. An association between HSV-1 and smoking with oral cancer development was suggested using immunologic parameters. Animal model studies using HSV-1 in rats have been suggestive as well.
Although the role of HPV in oropharyngeal cancer has been established, the role of HPV in oral cavity cancer at present is controversial. HPV-6 and HPV-16 have been most commonly implicated using polymerase chain reaction (PCR) assays of human tissue. In one study, HPV-16 was found to be present in tumor at a rate five times that of normal mucosa. Additionally, in one study oral squamous cell carcinoma cases were reported to have the presence of HPV. Studies reporting on 5-year survival have revealed an improved prognosis for patients with and without nodal disease who have HPV-16-positive tumors compared to those who lack HPV. Despite these positive studies, a number of institutions have reported minimal or no association with HPV and the oral cavity. Furthermore, a causative role has not been clearly established and questions have been raised regarding possible contamination of specimens and faulty analysis. At present, HPV’s role in cancer development of the oral cavity remains unclear.
Other Factors.
While causation has not been established, a number of other factors are found in higher degrees among patients with oral cavity cancer. Nutritional deficiency including iron and vitamins A, C, and E has been documented. A number of well-designed studies have suggested the protective benefits of diets high in total vegetable and fruit intake as well as fiber found in green vegetables. In a U.S.-based case-control study of second primary cancers, the risk of developing a second primary was 40% to 60% lower among those individuals with the highest levels of intake for total vegetables, demonstrating the protective nature of this diet.
Patients with oral cancer are found to have higher degrees of oral disease including periodontal disease and caries. While association is present, no direct causation can be attributed.
GENETIC ABNORMALITIES AND CLINICAL IMPLICATIONS
Chromosomal Abnormalities and Genetic Instability
Chromosomal abnormalities resulting from severe DNA damage have been extensively documented in head and neck squamous cell carcinoma (HNSCC). Abnormalities include both losses and gains in genetic material on chromosomes 3, 4, 7, 8, 9, 11, 13, 17, 18, and 19. Evidence suggests that some chromosomal alterations may be linked to malignant progression. For example, changes at 3p and/or 9p with any other chromosomal abnormality were associated with a 33-fold increase in risk of cancer.
Genetic instability includes microsatellite alterations and loss of heterozygosity. Microsatellites are highly polymorphic tandem repeat sequences of DNA that can be examined following amplification by polymerase chain reaction. Loss of heterozygosity (LOH) results from loss of one parent’s contribution to part of the cell’s genome. Both microsatellites and LOH are common findings in cancer. Microsatellites have been successfully used as tumor markers in a large number of malignancies including oral cancer. Using 52 known tumor-associated alterations in tumors compared to normal tissue in the same patient demonstrated 29% of patients to have at least 1 microsatellite marker present in tumor. Sidransky et al examined microsatellite markers or loss of heterozygosity in 21 patients, and showed that 86% (18/21) demonstrated LOH and 5 had both LOH and microsatellite alteration. Malignant progression has also been documented through microsatellite changes. From a clinical perspective, tetranucleotide microsatellite instability has been tested at surgical margins for recurrence prediction.
p53 ( TP53 Gene)
TP53 is located on the short arm of chromosome 17 at 17p13.1. It functions primarily to produce arrest of the cell cycle for DNA repair. Ultimately, if repair is unsuccessful p53 is one of the leading signals causing cells to engage in programmed cell death, or apoptosis, via a number of complex cellular signaling pathways. Mutations to TP53 are one of the most frequent abnormalities in HNSCC cell lines and tissue specimens, variably reported in 30% to 75% of tumors. Prognostic significance of p53 mutation has been reported. For example, node-negative oral squamous cell carcinoma patients with p53 overexpression were found to have poorer survival.
Epidermal Growth Factor Receptor (EGFR)
Epidermal growth factor receptor (EGFR) has been frequently found to be overexpressed in HNSCC. EGFR is a receptor tyrosine kinase that can be bound by ligands including EGF (epidermal growth factor) and transforming growth factor-alpha (TGF-α). Activation of EGFR leads to changes in a number of pathways including RAS and STAT for regulation of cell growth. EGFR mRNA is overexpressed in 92% of HNSCC with evidence of increased expression in advanced stage and poorly differentiated tumors. A number of studies have additionally documented the association of EGFR upregulation as an independent predictor for decreased disease-free survival. In addition, higher levels of EGFR have been noted in the oral cavity and pharynx compared to other HNSCC sites. Overexpression is the result of both decreased receptor down-regulation and increased mRNA synthesis. Association between EGFR activation and matrix metalloproteinase (MMP) has been suggested, lending evidence to EGFR’s role in invasion and metastasis.
A number of therapeutic strategies are in various stages of development to specifically target this receptor. Gefitinib (Iressa), a small-molecule EGFR-selective inhibitor of tyrosine kinase activity that blocks EGF autophosphorylation and activation, has shown limited success primarily because of its side effects and a lack of increase in patient survival. Preclinical models under current investigation include anti-EGFR variable regions VHH (nanobodies), immunoliposomes created with Fab′ fragments, and antisense or small interference RNA to EGFR.
In March 2006 the Food and Drug Administration approved Erbitux (cetuximab) for head and neck cancer as the first treatment in 45 years to increase survival when combined with radiotherapy for unresectable disease. Its approval followed trials that confirmed a survival advantage to patients with locoregionally advanced disease that underwent radiation therapy with cetuximab versus radiation alone.
Nuclear Factor-Kappa B
Nuclear factor-kappa B (NF-κB) is a transcription factor for inflammatory and immune response that serves as a cytokine modulator. In the normal cellular environment it remains in the cytoplasm tightly associated with protein inhibitors (I-κBs). With activation, I-κBs are phosphorylated and ubiquitinated with subsequent proteosomal degradation freeing NF-κB as a heterodimer of p65 and p50 subunits for nuclear translocation and gene activation. NF-κB is activated by a number of carcinogens and tumor promoters as well as the tumor necrosis factor (TNF) superfamily. Immunohistochemistry of biopsy specimens demonstrates that oral cancer expresses higher levels of NF-κB in comparison to normal and dysplastic tissue. Efforts to down-regulate NF-κB have been attempted using nonsteroidal anti-inflammatory drugs, which have been shown effective in vitro. To date no conclusive evidence to support this treatment exists in HNSCC.
Cyclin D1
Cyclin D1 is a regulatory protein that drives cells through the cell cycle. In HNSCC, cyclin D1 gene amplification has been recognized in 26% to 68% of cases. The presence of cyclin D1 overexpression has been correlated with increased nodal involvement, and decreased overall and tumor-free survival.
SCREENING FOR CANCER
Five-year survival of oral cavity cancer is inversely related to tumor size, the presence of nodal involvement, and distant metastasis. Detection in early stages has been shown to dramatically affect survival rates and is the most important variable leading to disease-free survival. Survival by stage for the oral cavity, reported by the American Joint Committee on Cancer, based on 1985 to 1991 data, is as follows: stage I, 65-70%; stage II, 50-55%; stage III, 38-44%; stage IV, 24-28%. While these numbers are somewhat lower than the currently reported statistics, their trend stands true. Given this reality, the desire for early diagnosis in order to increase survival has continued as a favorable objective. Overall, the success of the oral screening exam has been variably reported with sensitivities ranging from 57% to 61% and with a specificity of 98%. In 1965 Sandler reported success with visual inspection alone, in which 70 of 287 carcinomas and 20 of 28 carcinomas in situ were thought to be benign on clinical exam. Campaigns to enhance public and professional awareness are ongoing as a result of a number of studies that demonstrate both to be lacking. In addition, the desire for an early detection screening tool has led to the creation of adjuncts to the physical exam. At present, no accepted screening test is available and current guidelines from the American Cancer Society recommend only routine clinical examination of the oral cavity in association with the health maintenance exam. A number of limitations exist in the early detection paradigm worthy of limited exploration given the confines of this chapter.
Holmes and Dierks et al shed light on early detection in the United States, reporting their patient referral patterns for oral and oropharyngeal cancer. Fifty-one patients were reviewed with the conclusion that symptomatic and symptom-free detection was most likely to occur by a professional who was a regional specialist (routinely working in the oral cavity). Half of all patients who initially visited a non–regional specialist did not receive a biopsy or referral at their first visit regardless of the fact that they presented with later clinical and pathologically staged disease compared to those examined by a regional specialist. Since the majority of the regional specialists in the report were primary care dentists, it is important to consider the access of these professionals to the general public for the purposes of screening.
The 2003 National Health Care Disparities Report would suggest that less than 50% of all individuals more than 45 years of age see a dentist on an annual basis. By age 65 the estimated number is 42.6%. Outpatient visits to physicians for these same age groups are 77% and 89%, respectively. When these statistics are combined with the data from Holmes and colleagues, it is clear that barriers exist in access to the individuals most capable of early detection. If organized screening is to be effective it is likely that both provider and public awareness will need to be increased as well as access to care barriers addressed.
Until recently, no prospective data existed to validate mass screening for early detection of oral cavity cancer. In 2005 results from Kerala, India, offered the first support through prospective evaluation of over 191,000 patients. In the intervention group, visual examination was completed at 3-year intervals over a 10-year time period. The control group received no screening exams but did receive standard care. For those that developed cancer during the time of the study, the stage of disease in the intervention group was lower—41% stage I and II compared to 23% in the control, which was statistically significant. Overall 5-year survival was 50% in the intervention group compared to 34% in the control, which was again statistically significant. Mortality was decreased in the intervention group 21% overall, 22% for high-risk women, and 43% for high-risk men, with only the high-risk men group being statistically significant. Methodological weaknesses in the study have caused some to raise concerns as well as the fact that neither cost analysis nor harm from false-positive or false-negative exams is addressed.
DIAGNOSTIC ADJUNCTS
Because no true screening test other than oral exam exists at present, a number of adjuncts have been developed in an attempt to increase the sensitivity and specificity of the oral screening exam. These tests range from those with established records of utility to those recently introduced to the market for use.
Toluidine Blue
Toluidine blue is a metachromatic vital dye that binds nuclei with a high DNA or RNA content, such as may occur in precancerous (dysplastic) or cancerous lesions ( Figures 35-1 and 35-2 ). Its use as an adjunct to the physical exam for cancer screening has been documented over the last 3 decades with numerous reports in the literature. The sensitivity for toluidine blue screening has been reported to be 100% for lesions histologically consistent with invasive squamous cell carcinoma. In one study of 87 total lesions, sensitivity for invasive cancer was 100% (18/18); dysplasia revealed a less predictive result at 80% (29/39). The authors noted an advantage for toluidine blue as a screening tool because of the fact that a number of lesions not clinically visible before its application were discovered. Toluidine blue is limited by its specificity, which in this study was reported as 54%. Mashberg reported the ability to increase overall specificity with a two-wash technique in which positive results were followed by removal of irritants and repeat evaluation in 2 weeks. Using this technique he and his colleagues were able to raise specificities into the 90s. Onofre et al reported on their experience with 50 lesions in which sensitivity was 100% for carcinoma and carcinoma in situ. Dysplasia overall had a sensitivity of 50% with 3/6 mild dysplasias positive but 0/2 moderate dysplasias with uptake. Overall the specificity again was low at 67%. In the hands of the knowledgeable user, toluidine blue may offer an advantage over clinical examination alone. As a mass screening device, the lack of specificity results in a large number of false positives. In these cases clinical judgment would be required to decide whether or not biopsy or other diagnostic tests would be indicated.
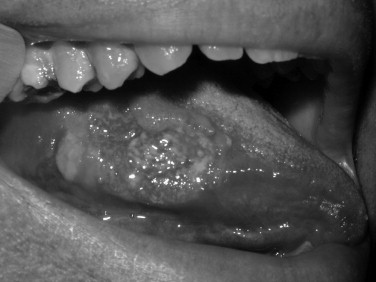
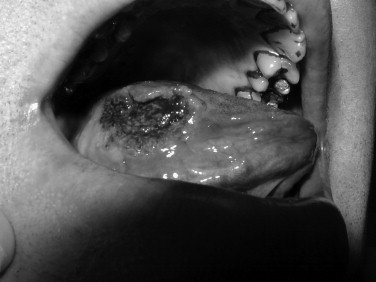
OralCDx (Brush Biopsy)
The brush biopsy was first reported via a multi-institutional randomized prospective trial in 1999. Unlike cytology, which has been shown ineffective for diagnosis of oral cavity cancer, this technique uses a brush, which has the capacity to procure a transepithelial specimen for microscopic evaluation of individual cells. The procedure is relatively painless and can be easily accomplished in a clinical setting. The results of the test are reported as positive, negative, and atypical. In the original report using 945 enrolled patients, a sensitivity of 100% was reported for 131 atypical and positive results, all of which revealed malignant or dysplastic lesions on histopathology. Specificity for positive results was reported as 100% and 92.9% for atypical results, with 14 of 196 total benign lesions testing atypical on brush biopsy. In addition, 4.5% of innocuous lesions that would not have been biopsied before brush were subjected after positive or atypical brush results and found to be malignant or dysplastic. A number of limitations to this study have been subsequently explored, which raise concerns for this technique. Because of institutional review board (IRB) restrictions and patient loss, 618 patients were never subjected to biopsy, which could have significantly altered the results. In clinical practice, concerns have been raised over the possibility of false-negative results, although the examples used in the only publication to raise these issues are of questionable appropriateness for a brush biopsy technique. Likely of greater clinical impact is the lower than expected positive predictive value of 38%, with 150/243 patients with positive or atypical results on brush being negative on scalpel biopsy. Two studies reported in Oral Oncology September 2004 highlight the continued debate of this technique. Poate et al reported on 112 patients with an overall sensitivity for dysplasia or cancer of 71.4% and a specificity of 32%. The positive predictive value of a positive or atypical result was 44% with a negative predictive value of 60%. In the same publication, Scheifele et al reported on 103 patients studied with OralCDx in which brush biopsy positive for dysplasia and cancer showed a sensitivity of 92% and a specificity of 94%. While some have argued these results as being superior to accepted screening tools in other body sites, such comparisons are unwarranted given significant differences in disease epidemiology and alternative screening methodologies for each disease.
Since clinical oral examination alone suffers in sensitivity but not in specificity and screening tests suffer from the opposite, a combination of these techniques in trained individuals may offer an advantage. Clinical judgment is necessary not only for conducting an appropriate visual examination but also for making determinations of which lesions to immediately subject to biopsy and which lesions in which an adjunct may be helpful, based on the pre-test probability of the patient and the specific adjunct characteristics relating to sensitivity and specificity. It is highly likely that additional tests will continue to be utilized and developed (e.g., VisiLite plus, VELscope, Microlux/DL). It is imperative that practitioners become proficient in evaluating the utility of screening tools and diagnostic adjuncts since these instruments may be of benefit to their patient population. Evaluating test characteristics utilizing the concepts of pretest probability, sensitivity, and specificity is critical to the practice of oral and maxillofacial surgery, now and in the future, in which an increasing number of diagnostic adjuncts are likely to become available. Science, and not marketing, should guide clinical practice.
EPIDEMIOLOGY
RISK FACTORS
Tobacco
Large, well-designed, population-based studies affirm the correlation between tobacco use and the risk of oral cavity cancer. Tobacco smoking is an independent risk factor; compared to nonsmokers, smokers are six to eight times more likely to develop oral cavity cancer. This is especially true for women, who are twice as likely as men to develop oral cancer given the same amount of tobacco consumption. More than 300 chemicals in tobacco smoke have been identified as contributing to carcinogenesis. It is thought that exposure to these carcinogens leads to malignant transformation of cells. Smoking cessation is effective in reducing risk, though not in its complete elimination. Individuals who refrained from smoking 1 to 9 years showed a 30% reduction of risk, with a 50% reduction for individuals abstaining from smoking more than 9 years.
Outside of cigarettes, both pipe and cigar smoking increase the risk for the development of oral cancer. The role of smokeless tobaccos within the United States has long been accepted to increase cancer risk, especially gingival and buccal cancers. A North Carolina-based study from 1986 showed a four times increase in risk in women users of smokeless tobacco. Recently some have argued the results of this study, claiming a lack of causation. Bouquot and Meckstroth reviewed statistics from West Virginia (the highest per capita consumption of smokeless tobacco) in regard to incidence and mortality of oral and pharyngeal cancer, concluding that rates were less than the national average. Accortt et al categorized 6779 subjects 45 to 75 years of age. In their study, exclusive use of smokeless tobacco was not shown to increase incidence of oral cancer in males or females. No synergistic effects were observed between smoking tobacco and smokeless tobacco. The use of smokeless tobacco, however, has been correlated with other cancer risk factors, with a recommendation for educating these patients regarding their overall cancer risk. It is likely that the debate of causation for smokeless tobacco and oral cancer will continue and patients should be aware at present that risks may exist.
Other forms of chewed tobacco have been shown as independent risk factors for oral cancer. Likely the most prominent is use of tobacco and betel nut in parts of India and Southeast Asia. In one study the incidence of oral cavity cancer was 123-fold higher for individuals who smoke and chew betel nut. These data infer that 40% to 50% of all cases of oral cavity cancer in this geographical region occur in individuals with this habit.
Alcohol
The relationship between alcohol and cancer is clear for hard liquor and high-level alcohol consumers but less well-defined in other risk groups. Approximately 75% of all patients who develop oral cancer do consume alcohol at some level. One Swedish-based study of more than 1300 individuals showed moderate alcohol intake alone (<10 g/day) did not increase risk significantly but high intake (>50 g) was an independent risk factor with a relative risk of 5.5. In addition, alcohol in conjunction with smoking was synergistic, with a relative risk of 22.1 compared to a relative risk of 6.5 from smoking alone. In another study looking at extreme alcohol consumers, the risk for cancer from alcohol has been found to be greater than that from smoking alone. Alcohol may predispose to cancer in any location in the oral cavity and is thought to possibly contribute more highly to dependent sites such as the floor of the mouth and lower buccal vestibule.
Viruses
A number of human viruses have been suggested as etiologic agents for oral cavity cancer through their transformation of human DNA. Herpes simplex virus type 1 (HSV-1) and human papillomaviruses (HPVs) have been reported by various authors. An association between HSV-1 and smoking with oral cancer development was suggested using immunologic parameters. Animal model studies using HSV-1 in rats have been suggestive as well.
Although the role of HPV in oropharyngeal cancer has been established, the role of HPV in oral cavity cancer at present is controversial. HPV-6 and HPV-16 have been most commonly implicated using polymerase chain reaction (PCR) assays of human tissue. In one study, HPV-16 was found to be present in tumor at a rate five times that of normal mucosa. Additionally, in one study oral squamous cell carcinoma cases were reported to have the presence of HPV. Studies reporting on 5-year survival have revealed an improved prognosis for patients with and without nodal disease who have HPV-16-positive tumors compared to those who lack HPV. Despite these positive studies, a number of institutions have reported minimal or no association with HPV and the oral cavity. Furthermore, a causative role has not been clearly established and questions have been raised regarding possible contamination of specimens and faulty analysis. At present, HPV’s role in cancer development of the oral cavity remains unclear.
Other Factors.
While causation has not been established, a number of other factors are found in higher degrees among patients with oral cavity cancer. Nutritional deficiency including iron and vitamins A, C, and E has been documented. A number of well-designed studies have suggested the protective benefits of diets high in total vegetable and fruit intake as well as fiber found in green vegetables. In a U.S.-based case-control study of second primary cancers, the risk of developing a second primary was 40% to 60% lower among those individuals with the highest levels of intake for total vegetables, demonstrating the protective nature of this diet.
Patients with oral cancer are found to have higher degrees of oral disease including periodontal disease and caries. While association is present, no direct causation can be attributed.
GENETIC ABNORMALITIES AND CLINICAL IMPLICATIONS
Chromosomal Abnormalities and Genetic Instability
Chromosomal abnormalities resulting from severe DNA damage have been extensively documented in head and neck squamous cell carcinoma (HNSCC). Abnormalities include both losses and gains in genetic material on chromosomes 3, 4, 7, 8, 9, 11, 13, 17, 18, and 19. Evidence suggests that some chromosomal alterations may be linked to malignant progression. For example, changes at 3p and/or 9p with any other chromosomal abnormality were associated with a 33-fold increase in risk of cancer.
Genetic instability includes microsatellite alterations and loss of heterozygosity. Microsatellites are highly polymorphic tandem repeat sequences of DNA that can be examined following amplification by polymerase chain reaction. Loss of heterozygosity (LOH) results from loss of one parent’s contribution to part of the cell’s genome. Both microsatellites and LOH are common findings in cancer. Microsatellites have been successfully used as tumor markers in a large number of malignancies including oral cancer. Using 52 known tumor-associated alterations in tumors compared to normal tissue in the same patient demonstrated 29% of patients to have at least 1 microsatellite marker present in tumor. Sidransky et al examined microsatellite markers or loss of heterozygosity in 21 patients, and showed that 86% (18/21) demonstrated LOH and 5 had both LOH and microsatellite alteration. Malignant progression has also been documented through microsatellite changes. From a clinical perspective, tetranucleotide microsatellite instability has been tested at surgical margins for recurrence prediction.
p53 ( TP53 Gene)
TP53 is located on the short arm of chromosome 17 at 17p13.1. It functions primarily to produce arrest of the cell cycle for DNA repair. Ultimately, if repair is unsuccessful p53 is one of the leading signals causing cells to engage in programmed cell death, or apoptosis, via a number of complex cellular signaling pathways. Mutations to TP53 are one of the most frequent abnormalities in HNSCC cell lines and tissue specimens, variably reported in 30% to 75% of tumors. Prognostic significance of p53 mutation has been reported. For example, node-negative oral squamous cell carcinoma patients with p53 overexpression were found to have poorer survival.
Epidermal Growth Factor Receptor (EGFR)
Epidermal growth factor receptor (EGFR) has been frequently found to be overexpressed in HNSCC. EGFR is a receptor tyrosine kinase that can be bound by ligands including EGF (epidermal growth factor) and transforming growth factor-alpha (TGF-α). Activation of EGFR leads to changes in a number of pathways including RAS and STAT for regulation of cell growth. EGFR mRNA is overexpressed in 92% of HNSCC with evidence of increased expression in advanced stage and poorly differentiated tumors. A number of studies have additionally documented the association of EGFR upregulation as an independent predictor for decreased disease-free survival. In addition, higher levels of EGFR have been noted in the oral cavity and pharynx compared to other HNSCC sites. Overexpression is the result of both decreased receptor down-regulation and increased mRNA synthesis. Association between EGFR activation and matrix metalloproteinase (MMP) has been suggested, lending evidence to EGFR’s role in invasion and metastasis.
A number of therapeutic strategies are in various stages of development to specifically target this receptor. Gefitinib (Iressa), a small-molecule EGFR-selective inhibitor of tyrosine kinase activity that blocks EGF autophosphorylation and activation, has shown limited success primarily because of its side effects and a lack of increase in patient survival. Preclinical models under current investigation include anti-EGFR variable regions VHH (nanobodies), immunoliposomes created with Fab′ fragments, and antisense or small interference RNA to EGFR.
In March 2006 the Food and Drug Administration approved Erbitux (cetuximab) for head and neck cancer as the first treatment in 45 years to increase survival when combined with radiotherapy for unresectable disease. Its approval followed trials that confirmed a survival advantage to patients with locoregionally advanced disease that underwent radiation therapy with cetuximab versus radiation alone.
Nuclear Factor-Kappa B
Nuclear factor-kappa B (NF-κB) is a transcription factor for inflammatory and immune response that serves as a cytokine modulator. In the normal cellular environment it remains in the cytoplasm tightly associated with protein inhibitors (I-κBs). With activation, I-κBs are phosphorylated and ubiquitinated with subsequent proteosomal degradation freeing NF-κB as a heterodimer of p65 and p50 subunits for nuclear translocation and gene activation. NF-κB is activated by a number of carcinogens and tumor promoters as well as the tumor necrosis factor (TNF) superfamily. Immunohistochemistry of biopsy specimens demonstrates that oral cancer expresses higher levels of NF-κB in comparison to normal and dysplastic tissue. Efforts to down-regulate NF-κB have been attempted using nonsteroidal anti-inflammatory drugs, which have been shown effective in vitro. To date no conclusive evidence to support this treatment exists in HNSCC.
Cyclin D1
Cyclin D1 is a regulatory protein that drives cells through the cell cycle. In HNSCC, cyclin D1 gene amplification has been recognized in 26% to 68% of cases. The presence of cyclin D1 overexpression has been correlated with increased nodal involvement, and decreased overall and tumor-free survival.
SCREENING FOR CANCER
Five-year survival of oral cavity cancer is inversely related to tumor size, the presence of nodal involvement, and distant metastasis. Detection in early stages has been shown to dramatically affect survival rates and is the most important variable leading to disease-free survival. Survival by stage for the oral cavity, reported by the American Joint Committee on Cancer, based on 1985 to 1991 data, is as follows: stage I, 65-70%; stage II, 50-55%; stage III, 38-44%; stage IV, 24-28%. While these numbers are somewhat lower than the currently reported statistics, their trend stands true. Given this reality, the desire for early diagnosis in order to increase survival has continued as a favorable objective. Overall, the success of the oral screening exam has been variably reported with sensitivities ranging from 57% to 61% and with a specificity of 98%. In 1965 Sandler reported success with visual inspection alone, in which 70 of 287 carcinomas and 20 of 28 carcinomas in situ were thought to be benign on clinical exam. Campaigns to enhance public and professional awareness are ongoing as a result of a number of studies that demonstrate both to be lacking. In addition, the desire for an early detection screening tool has led to the creation of adjuncts to the physical exam. At present, no accepted screening test is available and current guidelines from the American Cancer Society recommend only routine clinical examination of the oral cavity in association with the health maintenance exam. A number of limitations exist in the early detection paradigm worthy of limited exploration given the confines of this chapter.
Holmes and Dierks et al shed light on early detection in the United States, reporting their patient referral patterns for oral and oropharyngeal cancer. Fifty-one patients were reviewed with the conclusion that symptomatic and symptom-free detection was most likely to occur by a professional who was a regional specialist (routinely working in the oral cavity). Half of all patients who initially visited a non–regional specialist did not receive a biopsy or referral at their first visit regardless of the fact that they presented with later clinical and pathologically staged disease compared to those examined by a regional specialist. Since the majority of the regional specialists in the report were primary care dentists, it is important to consider the access of these professionals to the general public for the purposes of screening.
The 2003 National Health Care Disparities Report would suggest that less than 50% of all individuals more than 45 years of age see a dentist on an annual basis. By age 65 the estimated number is 42.6%. Outpatient visits to physicians for these same age groups are 77% and 89%, respectively. When these statistics are combined with the data from Holmes and colleagues, it is clear that barriers exist in access to the individuals most capable of early detection. If organized screening is to be effective it is likely that both provider and public awareness will need to be increased as well as access to care barriers addressed.
Until recently, no prospective data existed to validate mass screening for early detection of oral cavity cancer. In 2005 results from Kerala, India, offered the first support through prospective evaluation of over 191,000 patients. In the intervention group, visual examination was completed at 3-year intervals over a 10-year time period. The control group received no screening exams but did receive standard care. For those that developed cancer during the time of the study, the stage of disease in the intervention group was lower—41% stage I and II compared to 23% in the control, which was statistically significant. Overall 5-year survival was 50% in the intervention group compared to 34% in the control, which was again statistically significant. Mortality was decreased in the intervention group 21% overall, 22% for high-risk women, and 43% for high-risk men, with only the high-risk men group being statistically significant. Methodological weaknesses in the study have caused some to raise concerns as well as the fact that neither cost analysis nor harm from false-positive or false-negative exams is addressed.
DIAGNOSTIC ADJUNCTS
Because no true screening test other than oral exam exists at present, a number of adjuncts have been developed in an attempt to increase the sensitivity and specificity of the oral screening exam. These tests range from those with established records of utility to those recently introduced to the market for use.
Toluidine Blue
Toluidine blue is a metachromatic vital dye that binds nuclei with a high DNA or RNA content, such as may occur in precancerous (dysplastic) or cancerous lesions ( Figures 35-1 and 35-2 ). Its use as an adjunct to the physical exam for cancer screening has been documented over the last 3 decades with numerous reports in the literature. The sensitivity for toluidine blue screening has been reported to be 100% for lesions histologically consistent with invasive squamous cell carcinoma. In one study of 87 total lesions, sensitivity for invasive cancer was 100% (18/18); dysplasia revealed a less predictive result at 80% (29/39). The authors noted an advantage for toluidine blue as a screening tool because of the fact that a number of lesions not clinically visible before its application were discovered. Toluidine blue is limited by its specificity, which in this study was reported as 54%. Mashberg reported the ability to increase overall specificity with a two-wash technique in which positive results were followed by removal of irritants and repeat evaluation in 2 weeks. Using this technique he and his colleagues were able to raise specificities into the 90s. Onofre et al reported on their experience with 50 lesions in which sensitivity was 100% for carcinoma and carcinoma in situ. Dysplasia overall had a sensitivity of 50% with 3/6 mild dysplasias positive but 0/2 moderate dysplasias with uptake. Overall the specificity again was low at 67%. In the hands of the knowledgeable user, toluidine blue may offer an advantage over clinical examination alone. As a mass screening device, the lack of specificity results in a large number of false positives. In these cases clinical judgment would be required to decide whether or not biopsy or other diagnostic tests would be indicated.
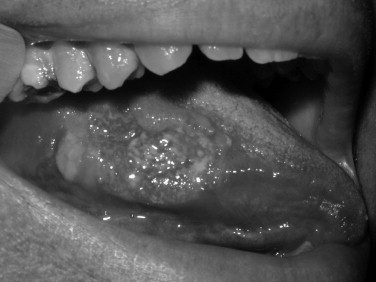
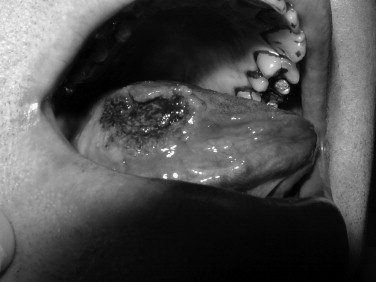
OralCDx (Brush Biopsy)
The brush biopsy was first reported via a multi-institutional randomized prospective trial in 1999. Unlike cytology, which has been shown ineffective for diagnosis of oral cavity cancer, this technique uses a brush, which has the capacity to procure a transepithelial specimen for microscopic evaluation of individual cells. The procedure is relatively painless and can be easily accomplished in a clinical setting. The results of the test are reported as positive, negative, and atypical. In the original report using 945 enrolled patients, a sensitivity of 100% was reported for 131 atypical and positive results, all of which revealed malignant or dysplastic lesions on histopathology. Specificity for positive results was reported as 100% and 92.9% for atypical results, with 14 of 196 total benign lesions testing atypical on brush biopsy. In addition, 4.5% of innocuous lesions that would not have been biopsied before brush were subjected after positive or atypical brush results and found to be malignant or dysplastic. A number of limitations to this study have been subsequently explored, which raise concerns for this technique. Because of institutional review board (IRB) restrictions and patient loss, 618 patients were never subjected to biopsy, which could have significantly altered the results. In clinical practice, concerns have been raised over the possibility of false-negative results, although the examples used in the only publication to raise these issues are of questionable appropriateness for a brush biopsy technique. Likely of greater clinical impact is the lower than expected positive predictive value of 38%, with 150/243 patients with positive or atypical results on brush being negative on scalpel biopsy. Two studies reported in Oral Oncology September 2004 highlight the continued debate of this technique. Poate et al reported on 112 patients with an overall sensitivity for dysplasia or cancer of 71.4% and a specificity of 32%. The positive predictive value of a positive or atypical result was 44% with a negative predictive value of 60%. In the same publication, Scheifele et al reported on 103 patients studied with OralCDx in which brush biopsy positive for dysplasia and cancer showed a sensitivity of 92% and a specificity of 94%. While some have argued these results as being superior to accepted screening tools in other body sites, such comparisons are unwarranted given significant differences in disease epidemiology and alternative screening methodologies for each disease.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


