how to maintain tissue architecture
Fausto Frizzera, Vítor M. Sapata, Ronald E. Jung, Elcio Marcantonio Jr, Jamil A. Shibli

1. INTRODUCTION
The health of the population may be affected by tooth loss, which can cause esthetic and functional changes, in addition to compromising quality of life1,2. Extraction leads to a process of bone remodeling in the alveolar ridge, where part of its initial architecture is lost3,4. The ridge architecture can be preserved through gingival and bone grafting, allowing the rehabilitation of the lost tooth with esthetic and functional implant treatment.
The teeth are in close relationship with the alveolar ridge and its extraction promotes changes in the shape of the ridge5,6. To preserve the tissue, the implant is placed and a provisional installed at the moment of the extraction7,8. The final result is dependent on tissue reconstruction and correct implant positioning, which requires excellent surgical precision9. A systematic literature review showed no clinical or biologic differences between the techniques used for single rehabilitation with implants10.
Although not demonstrating a significant difference between approaches, the studies do not take into account one crucial factor: the patient’s desire to receive a fixed implant rehabilitation in a shorter time frame. Immediate rehabilitation is a technique with high technical demands, which depends on patient cooperation and is often not routinely performed by clinicians. The literature tends to favor a delayed approach, where the socket is first grafted, and in a second surgical procedure, the implant is placed11.
OBJECTIVES
At the end of this chapter, the reader should be able to:
-
Know the clinical and biologic events that occur after extraction.
-
Select the biomaterials recommended to minimize bone remodeling.
-
Use soft tissue grafts to compensate for volumetric ridge change.
2. EVIDENCE BASED ON THE LITERATURE
2.1. BONE CHANGES AFTER EXTRACTION
Socket remodeling begins immediately after tooth removal. Then a series of biologic events will occur, resulting in the filling of the socket, which loses part of its total volume. Studies in animals showed that the bone socket loses about 35% of its volume after an extraction12,13. In humans, approximately 50% of the thickness of the ridge is lost and may exceed 4 mm of horizontal reduction in the first 6 months14–17. Depending on the architecture of the ridge, the remaining bone may be insufficient for implant placement in the optimal position (Figs 01A–R). This remodeling can lead to esthetic difficulties or the need for grafts, thereby causing higher morbidity18. Socket grafting attempts to avoid the tissue resorption that occurs naturally after extraction due to loss of function and nutrition to the bone.


















01. A–R Biologic events that occur after extraction: sagittal (A–F), frontal (G–L), and transverse (M–R) planes. Reducing the socket volume may impair future implant placement.
The extraction must be a minimally traumatic procedure to prevent or decrease the alveolar bone loss that occurs after extraction. Gingival detachment should be performed with a scalpel blade or delicate periosteal elevator. The instrument used for the extraction will depend on the remaining tooth structure (Figs 02 to 05A–F). Devices such as the tooth extraction system, modified forceps, and periotomes can be used for this purpose19. Maintenance of the socket architecture also depends on the preservation of its walls. The use of grafting material facilitates socket preservation20–23.

02. Devices used for minimally traumatic extraction. In the presence of root tips, the dental extraction system and periotomes may be used. If the crown is intact, extraction may be performed with a periotome; in conical or circular roots extraction, forceps can be used by gentle rotational motion.






03. A–F The dental extraction system works by preparing the root canal with a bur, screwing in the device for extraction, and removing the root by means of a wire and pulley system.






04. A–F Extraction with a periotome works by inserting the instrument in the periodontal ligament and using a wedge movement on the proximal and palatal surfaces. Flexible periotomes allow a slight rotational movement to dislocate the tooth or root. After rupture of the periodontal fibers, the remaining tooth structure should be carefully removed.






05. A–F For extraction with forceps, the instrument needs to be adapted at the cervical area and a gentle rotation movement must be performed. Rupture of the periodontal fibers with this movement allows the tooth to be removed.
Socket repair occurs even in the absence of grafts, where the blood clot will regulate the process of bone remodeling. Araújo and Lindhe3 described the three phases of the histologic changes occurring after extraction:
2.1.1. INFLAMMATORY PHASE
Early after extraction, blood from the alveolar walls forms a clot to stop the bleeding. This clot is gradually replaced by a granulation tissue composed of fibroblasts, vascular structures, and inflammatory cells that migrate to the socket to remove impurities and microorganisms. During this phase, osteoclasts are present inside and outside the socket, especially on the cancellous and bundle bone.
2.1.2. PROLIFERATIVE PHASE
The maintenance of cell types present in granulation tissue leads to collagen production and vascular neoformation. A provisional matrix is formed, which will be mineralized later. Osteoblasts stay close to the newly formed bone tissue, where they are linearly grouped. In this phase, the primary bone that fills the socket is formed. This bone has low mechanical strength. Tissue mineralization occurs from the lateral walls of the socket and toward its center, as well as in the most apical portion of the socket, followed by the central and coronal portion.
2.1.3. MODELING AND REMODELING PHASE
In a more advanced phase of the alveolar healing process, it is possible to verify the formation of a more significant amount of secondary and medullary bone, as well as limited areas of osteoclast-mediated bone resorption in the Howship lacunae. Bone remodeling is known as bone turnover, where bone resorption and formation processes occur together to renew and maintain the tissue in homeostasis. Alteration of the alveolar ridge shape is called bone modeling and can be verified mainly in the buccal wall, both in height and thickness3.
The alveolar process undergoes several tissue changes in the first year after extraction. The highest amount of bone loss is concentrated in the first 3 months6. When the buccal bone wall has a thickness of 2 mm or more, there is reduced remodeling. However, this only happens in around 2.6% of the anterior teeth. The average thickness of the buccal bone wall tends to be less than or equal to 0.5 mm24–27.
2.2. SOCKET PRESERVATION
After extraction, a horizontal reduction of the alveolar ridge between 2.6 mm and 4.6 mm is expected11,22,28,29. Socket preservation is recommended to limit this remodeling. The technique consists of filling the socket with grafting material, reducing its loss to between 0.5 mm and 1.5 mm20–23,29–31 (Figs 06A–D).




06. A–D Dry skull image with post-extraction alveolar bone remodeling on tooth 21 (image taken at the Anatomy Laboratory of Faesa University) (A). Clinical appearance of a fresh socket and healed ridge, demonstrating the intensity of ridge remodeling (B). Occlusal view of the central incisor region with a buccal bone defect where only extraction (C) and extraction associated with socket preservation were done (D).
Osteointegration of the graft will provide incorporation and union between the grafted material and bone neoformation32. Its occurrence and the formation of new bone depend on the mechanism of action of the chosen graft (Tables 01, 02, and Figs 07A–H). There are three fundamental elements to bone regeneration: osteogenesis, osteoinduction, and osteoconduction. The autogenous bone graft is the only one that presents all three elements. For small and medium defects, intraoral donor areas are recommended, usually the ramus, tuberosity, chin, or palate. For extensive reconstructions such as severely resorbed jaw or bone defects caused by tumors or trauma, removal of a block graft from extraoral regions such as the skull, iliac bone, tibia, and rib may be necessary, as well as intraoral regions. However, the use of block grafts is becoming very restricted due to the evolution of biomaterials.

Table 01. Grafts can be classified according to their origin33
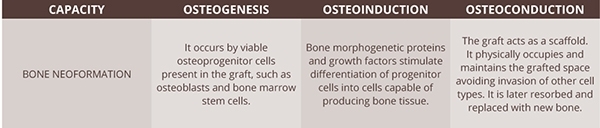
Table 02. Bone grafts can influence new bone formation in three ways








07. A–H Grafts that can be used for guided bone regeneration. Autogenous block-shaped graft (A), large particle xenograft (B), and small particle alloplastic graft (C); a xenograft may also be a combination of material from animals of different species (bovine inorganic graft plus 10% porcine collagen) (D) hydrated with serum (F, G) and blood (H).
2.2.1. AUTOGRAFTS AND ALLOGRAFTS
Autogenous bone is considered a gold standard, although it has higher morbidity than other grafts and limited effectiveness in maintaining the architecture of the dental socket. Araújo et al34 demonstrated no histologic difference in healing between grafted and non-grafted sockets using autografts. Research also showed that an autograft did not favor alveolar bone repair and was ineffective in maintaining the shape of the ridge, with a reduction of approximately 2 mm in the height of the buccal crest and 25% of the ridge volume.
Demineralized, frozen, and dried bone allograft and frozen and dried bone allograft demonstrate excellent results in maintaining socket volume35,36. However, this material cannot be commercialized in some countries due to laws prohibiting the commercialization of human tissues. In contrast, there is a limited number of scientific papers proving the predictable use of allografts from national bone banks.
2.2.2. XENOGRAFTS
Xenografts were first used decades ago to reduce bone turnover37. The inorganic bovine bone, a slow resorption osteoconductive material, is the most commonly used xenograft38. Studies about this graft in humans and animals show its effectiveness in maintaining the alveolar ridge shape, implant osseointegration, absence of inflammatory reactions, and gradual resorption of its particles, mostly surrounded by bone tissue37,39,40–42.
The incorporation of 10% of purified porcine type I collagen into inorganic bovine bone gave the product its trading name (BOC). The addition of collagen promotes cohesion between the particles of the biomaterial, facilitating manipulation and incorporation into the receptor area. The biomaterial preserves alveolar architecture, reducing the amount of bone loss and is superior to extraction without grafting4. Numerous studies have been conducted in humans and animals proving its efficacy compared to other bone grafts43–46.
The use of inorganic bovine bone combined with porcine collagen in a fresh socket increases the amount of bone formation. It can maintain the alveolar architecture, demonstrating the benefits of using this graft when compared to non-grafted sockets. Additionally, approximately half of the alveolar ridge of non-grafted sockets is composed of bone marrow. In contrast, in those grafted with the biomaterial, this amount represented only 27%. Following the use of BOC in fresh sockets, the formation of dome-shaped mineralized bone tissue was verified and the new bone formed was in direct contact with the biomaterial, the lingual and buccal bone wall12.
Histologically, in the initial weeks after extraction and grafting with inorganic bovine bone plus 10% porcine collagen, the portion corresponding to collagen is reabsorbed and alveolar remodeling occurs gradually. The bone graft has an osteoconductive function. It maintains the framework to allow the migration of cells of bone tissue and provide bone neoformation. As the biomaterial is reabsorbed, new bone tissue is formed. Part of this material can still be verified in histologic evaluations years after the grafting procedure, which characterizes it as a slow resorption material4.
The use of inorganic bovine bone in a bone defect that has all walls, such as in a socket, leads to the formation of hard tissue. However, alveolar healing time is increased47–50. Comparative studies in humans showed that when an inorganic bovine bone was used in the socket, maintenance of the alveolar architecture was more significant than when it was not used43,51,52.
2.2.3. SYNTHETIC GRAFTS
Synthetic grafts are available and are cheaper, but their efficacy is questioned. A systematic review of the literature showed that autografts and xenografts have better results in post-extraction compared to synthetic grafts or non-grafted sockets. Histologically, alloplastic grafts presented a significant amount of vital bone, a smaller amount of biomaterial, and connective tissue. However, among the evaluated grafts, they presented the highest resorption rate and loss of height and thickness of the alveolar ridge53.
2.2.4. MEMBRANES
Usually, if defects are present in the walls, placement of grafts and membranes is indicated to prevent the occurrence of alveolar defects, making future implantation complicated3,54,55. Studies that evaluated the filling of this space with such a combination showed a more significant amount of bone formation and preservation of bone architecture4,44,56,57.
Treating defective sockets requires a different approach than alveolar preservation because tissues need to be reconstructed. The socket (Figs 08A–I) can be classified into55:
-
Type I: When bone and gingival tissues are intact.
-
Type II: There is a buccal bone defect, but the gingival margin is properly positioned.
-
Type III: There is a buccal bone defect and gingival margin recession.









08. A–I Types of socket: I intact; II with a bone defect; and III with a bone and gingival defect.
In esthetic areas, bone loss requires tissue reconstruction using membranes, bone, and gingival grafts55. Membranes function as a barrier, keeping the grafted material inside the socket, preventing graft particles from lodging into the gingival tissue, and migration of soft tissue cells into the grafting material58–60. To reduce surgical and biologic trauma to the remaining bone walls, periosteum detachment should be avoided. The membrane should be cut and adapted according to the existing defect and slightly positioned inside the socket, covering 1–2 mm of bone55. Bone cells will migrate through the resorbable membrane and other walls of the socket to allow graft incorporation. Membrane resorption time, and the number of cells and vessels that will pass through it, will depend on their physicochemical characteristics.
Most membranes used in dentistry are of xenogenous or synthetic origin. One of the essential precautions regarding their use is to prevent them from being exposed to the oral environment since exposure leads to contamination of the grafted material by oral bacteria, resulting in a decrease in regenerated tissue61,62. The first membranes used were nonresorbable and had a high exposure index, requiring a second surgery to remove them. For these reasons, their use was severely reduced after the development of resorbable membranes (Figs 09A–K). These membranes are safe and predictable when performing guided bone or tissue regeneration, as demonstrated by animal and human studies63–66. Even when exposed, the soft tissue usually has no infection because exposed collagen is easily degraded67,68.











09. A–K Synthetic membrane (A) that can be cut in the form of an “ice cream cone” (B) or cone (C) and has greater consistency after hydration. Membrane of porcine origin (D) that can be cut according to the defect and has better adaptation after hydration (E). Cone-shaped membrane adaptation for correction of buccal bone defect and filling of the socket with large particle xenograft (F–K).
Reabsorption of collagen membranes is associated with their chemical processing, the type of tissue, and the cross-linking collagen69. Membranes can be classified into cross-linked or non-cross-linked based on this structure. Membranes with more cross-links are more difficult for the organism to resorb. One study that compared the use of these two membrane types in peri-implant defects demonstrated that cross-linked membranes have more exposure than non-cross-linked membranes because they trigger a higher immune-mediated inflammatory response64.
Although collagen membranes have a faster rate of degradation, they have the capacity for hemostasis, clot stabilization, and semipermeability, allowing nutrient transfer to the grafted area70,71. Formation of new vessels precedes bone neoformation. These processes are closely related, so it is essential to use a membrane that allows early angiogenesis60.
Geistlich Bio-Gide is a non-cross-linked, double-layered native porcine collagen type II membrane. The smooth layer should face the flap and the porous layer should be in contact with the grafted area72,73. The fact that it has a faster resorption rate than cross-linked membranes is not a concern since most tissue is newly formed in the early weeks after the grafting procedure74,75. Schwarz et al71 evaluated the immunohistochemical and histologic characteristics of the angiogenesis of cross-linked and non-cross-linked membranes in rats. The results of this study proved the superior capacity of angiogenesis through the native collagen membrane compared to the other cross-linked membranes.
One study demonstrated that the combination of native collagen membrane with inorganic bovine bone and repositioned flap allowed the formation of a higher bone volume compared to a graft without a membrane. Flaps improperly performed on esthetic areas may compromise the results of treatment and should be carefully planned76.
Performing grafts with a buccal bone defect without a flap allows a better postoperative period for the patient by reducing pain and edema, decreasing the amount of bleeding and surgery time, and preventing the formation of tissue scarring. It does not alter the position of the mucogingival line, favoring nutrition of the bone remnant and graft, besides preserving the architecture of the ridge15. An advantage of using the membrane on the buccal surface is that it allows the graft to be adequately compressed so as to push the buccal soft tissue to maintain the contour of the ridge (Figs 10A–F).






10. A–F Compromised socket that received grafting with a native collagen membrane and inorganic bovine bone plus porcine collagen. Note the possibility of projecting the tissue volume to the buccal side with the use of a membrane and maintaining the volume obtained after its healing.
Due to the characteristics of the bone graft used, alveolar remodeling is dependent on the remaining bone after extraction. A socket with a bone defect will have a different bone remodeling pattern than the intact socket, presenting a more significant loss of the alveolar area77. A study in primates evaluated the use of a metallic device (SocketKAGE) to stabilize the alveolar structure with complete loss of the buccal bone wall associated with inorganic bovine bone or clot77. One year after surgery, use of the device combined with a bone graft showed better results in bone height and thickness compared to the other group.
Tan-Chu et al15 evaluated the contour of the alveolar ridge after treating sockets with buccal bone defect using the “ice cream cone” technique described previously55. The lower part of the membrane was cut according to the buccal defect (similar to a cone). The upper part was cut according to the entrance of the socket (similar to ice cream) (Figs 11A–I and 12A–N). After tomographic analysis of ridge thickness, intraoral scanning, and measurement of the cast model, an average loss of 1.32 mm in thickness was observed 6 months after surgery. Sufficient buccal wall and the remaining bone structure allowed for implant placement15.









11. A–I Adaptation of the “ice cream cone” membrane to correct a buccal bone defect and filling the socket with a large particle xenograft. Sealing the socket is performed by folding and suturing the membrane over it.



12. A–C Sequence of images for alveolar extraction and preservation by “ice cream cone” technique (A–C)..











12. D–N The socket should be curetted, irrigated, and the inner edges of the soft tissue should have the epithelium removed (D–N)The height and width of the socket should be measured so that the membrane is cut according to the size of the defect (F–I). The membrane and bone graft must be inserted into the socket and a suture is used to stabilize the grafting material (J–N).
2.3. SOCKET SEALING WITH SOFT TISSUE GRAFT
The socket submitted to the alveolar preservation technique should receive a bone graft and cervical sealing with a membrane or soft tissue graft to prevent graft particle migration and contamination of the grafted area, also allowing an increased volume of gingival tissue78–80. A buccal flap is not indicated unless there is root involvement that was not determined after radiographic and clinical examinations, culminating in the need for exploratory surgery to close the diagnosis. Elevation of the buccal flap and closure of the socket by releasing this flap is not recommended because of increased morbidity, a decrease in the amount of attached gingiva, changes in the position of the mucogingival junction, and damage to the buccal bone wall81. Autogenous, xenogenous, or allogenous soft tissue graft can be used as a socket seal. The gingival grafts (subepithelial connective tissue and connective tissue combined with epithelium) and collagen matrix (Figs 13A–F) are the most commonly used.






13. A–F Types of socket preservation with different types of grafts depending on the integrity of the alveolus (A) and papillae. Socket sealed with collagen matrix (B) and epithelium-connective tissue graft (C). Sealed buccal bone loss with connective tissue graft (D), collagen matrix (E), and epithelium-connective tissue graft (F).
2.3.1. GINGIVAL GRAFTS (EPITHELIUM-CONNECTIVE TISSUE AND SUBEPITHELIAL CONNECTIVE TISSUE)
Autogenous gingival grafts are usually removed from the hard palate, maxillary tuberosity, or edentulous areas. The surgical technique for removal and tissue content are the differences between the epithelium-connective tissue and subepithelial connective tissue grafts (Figs 14A–I).









14. A–I Removal of epithelium-connective tissue graft. A guide with the diameter of the socket is made with a suture. Using a delicate scalpel blade, the donor area is traced, the guide is then removed and a 2–2.5-mm-deep incision is made around the traced area (A–C). Graft removal is performed with an incision parallel to the periosteum (D–F). The donor area is then sutured and protected with a mechanical barrier. The graft is tested in the socket to verify the need for size adjustment (G–I).
 VIDEO OF SOCKET PRESERVATION WITH EPITHELIUM–CONNECTIVE TISSUE GRAFT
VIDEO OF SOCKET PRESERVATION WITH EPITHELIUM–CONNECTIVE TISSUE GRAFT
These grafts can be used to: increase the attached gingiva; increase tissue thickness on flanges, teeth, or implants; coverage of Miller class I and II gingival recession; maintenance of gingival margin position in the socket when associated with immediate implant, provisional, and biomaterials; and alveolar sealing where socket preservation is used82–85 (Figs 15A–F to 18A–L).






15. A–F After graft testing and adaptation, simple sutures are performed to stabilize and coaptate the edges.





16. A–E Patient sample from clinical research conducted by Dr. Jamil Shibli, where alveolar preservation with recombinant human bone morphogenetic protein-2 and free gingival graft was performed. Study performed by Dr Leda Marina Lima and Walterson M. Prado.





17. A–E In this research, volumetric evaluation of the reconstructed ridge with this material gave satisfactory results, with greater volume loss in the external 5 mm of the alveolar ridge in the vestibular region. Study performed by Dr Leda Marina Lima and Walterson M. Prado.












18. A–L After removal and testing of the connective tissue, a stabilizing suture on the buccal and lingual is performed. If necessary, simple sutures can be made by attaching the graft to the gingival tissue for better coaptation.
These grafts increase morbidity by creating a new surgical area that can have complications during its removal and healing (Figs 19A–U to 26A–I)86. Another disadvantage when working with autogenous grafts is due to the limited amount of donor tissue (Tables 03 and 04)82.





















19. A–U Patient with a gummy smile and history of trauma in the region of the maxillary central incisors. Before orthodontic treatment, tooth 11 had internal resorption. Orthodontic treatment was approved and followed by the endodontistin charge of treatment. During treatment, the tooth presented a fistula and a periodontal pocket (A–M). Minimally traumatic extraction of tooth 11 and socket preservation (N–P). The granulation tissue was removed and the socket irrigated with saline; then, the socket was inspected with a periodontal probe, which showed extensive loss in its distal portion (Q–U).

























20. A–Y The socket margins were de-epithelized with a bur and a fistulectomy was performed with the appropriate instruments (A–D). Socket preservation was performed with Bio-Oss Collagen; the epithelium-connective tissue graft was removed from the palate with a scalpel and 15C blade (E–N). To protect the donor area, a removable acrylic orthodontic appliance and surgical cement were used. In the recipient area, the gingival graft was stabilized at the gingival margins using simple sutures (O–S). Postoperative condition after 14 days presented a good integration between graft and socket (T–Y).









21. A–J Four months after socket preservation, the results were verified and flapless surgery was performed to place the implant (A–H). A circular scalpel was used to allow access to the ridge and a 3.5 × 11 implant (Drive Acqua; Neodent) installed in the ideal three-dimensional position, considering the result from orthodontic treatment (I, J).









22. A–I The area was prepared and received a connective tissue graft removed from the tuberosity region (A–E). Sutures were removed 7 days after surgery. A tomography was performed to confirm correct implant placement (F–I).






23. A–F Digitally planned image demonstrating the need to intrude the maxillary incisors and crown lengthening on the incisors (A). Appearance after provisional installation tooth 11 (B, C) and orthodontic appliance removal (D). Final outcome after treatment (E, F). Orthodontic procedure: Deise Cunha; surgical procedure: Dr Fausto Frizzera; restorative procedure: Dr Marco Masioli; laboratory technician: Igor Hand.










24. A–J Presence of buccolingual vertical fracture in tooth 25 (A–F). Due to the presence of an extensive lesion and loss of interproximal tissue, a flap for access, debridement, and grafting of the region with large particles of inorganic bovine bone graft was performed (G–J).






25. A–F The grafted area was protected with an acellular dermal matrix and a connective tissue graft was stabilized over the ridge to facilitate flap closure and increase volume in the free and interproximal surfaces (A, B). Clinical appearance after 15 (C, D) and 180 days (E, F).









26. A–I A graft tissue biopsy was performed and flapless implant (A–G) placement surgery was done. The histologic analysis revealed remodeling of the biomaterial and its intimate contact with the vital bone tissue (5× and 40×) (H, I). Surgical procedure: Dr Elcio Marcantonio; restorative procedure: Dr Rogério Margonar.
|
TYPE OF GINGIVAL GRAFT: ADVANTAGES |
EPITHELIUM–CONNECTIVE TISSUE |
SUBEPITHELIAL CONNECTIVE TISSUE |
|---|---|---|
|
Increase in tissue thickness |
+ |
+++ |
|
Increase in attached gingiva |
+ + + |
+ |
|
Coverage of gingival recession |
* |
+++ |
|
Maintenance of gingival margin post-extraction |
* |
+++ |
|
Socket sealing |
+ + |
+ |
|
*Not indicated |
Table 03. Comparison of the results obtained between the various indications for autogenous gingival grafts
|
TYPE OF GINGIVAL GRAFT: ADVANTAGES |
EPITHELIUM–CONNECTIVE TISSUE |
SUBEPITHELIAL CONNECTIVE TISSUE |
|---|---|---|
|
Graft removal |
++ |
+ |
|
Time for graft removal |
+ |
++ |
|
Morbidity |
+++ |
+ |
|
Need for protection of the donor area |
+++ |
+ |
|
Color and texture of grafted area |
– |
+++ |
Table 04. Comparison between gingival grafts with regard to their main disadvantages
2.3.2. COLLAGEN MATRIX GRAFT
The three-dimensional collagen matrix (Geistlich Mucograft; Straumann Mucoderm) has a xenogenous origin and consists of two layers. One compact layer, which helps structural maintenance of the graft and facilitates its suturing and cell adhesion, and one porous layer, which aids fluid absorption, clot organization, and graft integration into the recipient bed (Figs 27A–S). Few studies have evaluated its use. Human and animal studies have been conducted and have demonstrated the effectiveness of this collagen matrix to treat areas with no or limited amount of attached gingiva87,88, increased alveolar ridge volume89,90, treatment of Miller class I and II gingival recession39, and socket sealing91 and may also be used as a dermal substitute92. Histologically, in 3–4 months the graft was incorporated, showing no histologic differences when compared to a non-grafted area or one that has received a free gingival graft88,90.



















27. A–S Tooth 14 presented extensive subgingival caries, a fracture in the pulpal floor, gingival recession, and absence of buccal bone (A–C). Minimally traumatic extraction was performed. The socket was grafted with a collagen membrane on the buccal surface. A slow resorption graft biomaterial and sealing with porcine collagen matrix (D–I) were used. Four months after alveolar preservation, soft tissue height gain and buccal bone regeneration were verified; surgery was performed to remove bone for biopsy and install a 3.5 × 11 implant (Drive Acqua; Neodent) (J–Q). Histologic analysis (R, S) showed slow remodeling of the biomaterial and intimate contact with vital bone tissue (20× magnification). Surgical procedures: Dr Keila Soares and Dr Mariana Buaiz with orientation from Dr Fausto Frizzera.
3. CLINICAL APPLICATION
The literature presents several materials and techniques used to change the quantity or quality of tissues. However, a grafting material that provides satisfactory results with minimal biologic cost has yet to be deemed as ideal90. The material of choice for alveolar bone grafting is inorganic bovine bone containing 10% porcine collagen. Although autogenous tissue grafts are considered the gold standard, socket preservation has a number of disadvantages such as increased morbidity and surgical time. Allografts are banned from commercialization in some country and ethical issues regarding their use and potential for disease transmission remain87.
The use of heterografts has a number of advantages compared to other types of grafts.
When considering the different types of sockets, grafts, and clinical situations, a unified treatment protocol for all cases is difficult to establish (Tables 05, 06 and Figs 28A–L to 33A–H). To facilitate socket preservation aimed at maintaining bone volume, a surgical technique was proposed for esthetic areas and is shown on the right.

Table 05. Flowchart for socket treatment when an immediate implant is not recommended

Table 06. Decision-making regarding the type of gingival graft to be used for socket sealing
SURGICAL TECHNIQUE
-
Anesthesia of the operative region
-
Minimally traumatic extraction with the appropriate devices
-
Clockwise or counterclockwise curettage of the entire socket
-
Abundant irrigation with saline
-
Inspection of the socket with a periodontal probe to assess the remaining bone on the free and interproximal surfaces*
-
Removal of the epithelium from the edges of the alveolus with a blade or drill
-
Alveolus grafting with resorption biomaterial and membrane if necessary
-
Sealing of the alveolus with gingival graft
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


