Fig. 3.1
Intraoral photographs. (a1–a3) Pretreatment. (b1–b3) After a year of active treatment, upper and lower MEAW (multiloop edge-wise arch wires) were placed and 3/16-inch, 6-oz vertical and class III elastics were applied. (c1–c3), (d1, d2,) Posttreatment. The active treatment duration was 32 months
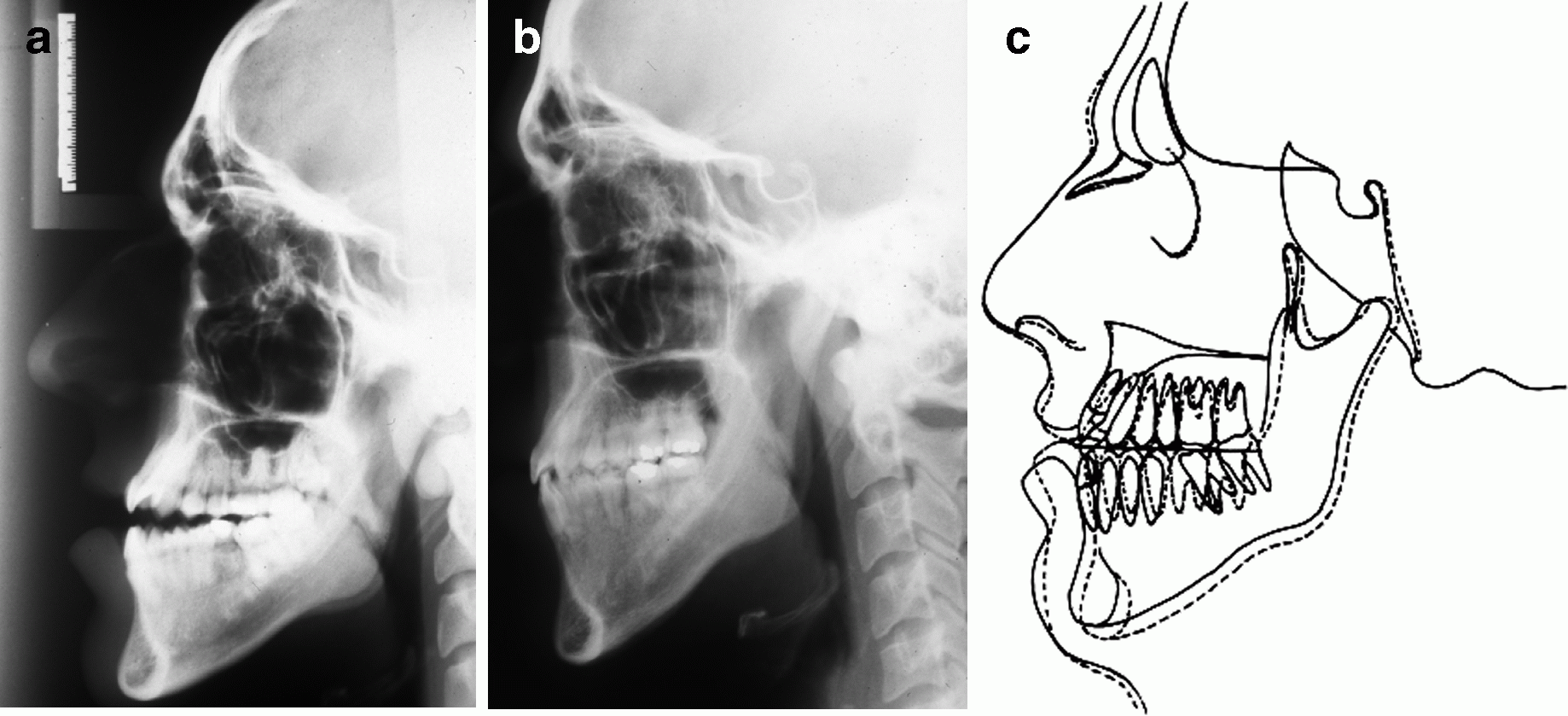
Fig. 3.2
Cephalometric radiographs. (a) Pretreatment. (b) Posttreatment. (c) Pre-/posttreatment superimposition
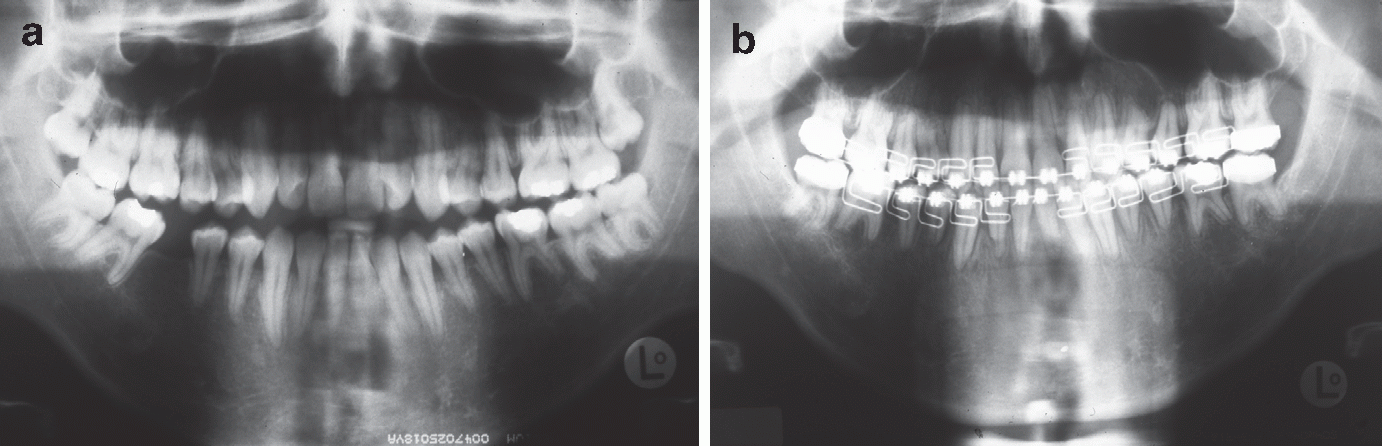
Fig. 3.3
Pantomographs. (a) Pretreatment. (b) Just before appliance removal
Accordingly, it was postulated that the impairment of perception of the teeth may indicate that in the open bite the sensory input, stemming at least from the periodontal mechanoreceptors of the incisor teeth, was reduced resulting in sensory deprivation of the somesthetic cortex, where the oral sensory system is point-to-point represented and perceived. This view, is consistent with the studies of Morimoto and Kawamura [88] who found that subjects with a normal occlusal relationship of their incisor teeth could discriminate a 0.2-mm difference in the size of wires held between the teeth, while subjects with maloccluded incisor teeth needed a difference of more than 0.4-mm in wire size in order to discriminate them. They concluded that periodontal receptors have reduced sensibility in malopposed teeth.
Similarly, the studies of Teuber et al. [89] and Haber [90] suggest that changes in perceptual function may happen in limited areas of the somesthetic cortex whose sensory input has been reduced as a result of sensory or perceptual isolation due to loss of a limb. The amputation of a limb affects the regulatory function of sensory input exerted by the ARAS in the cortex, resulting in impairment of limb perception.
However, an alternative hypothesis concerning impairment of consciousness of the presence of the incisor teeth in open bite malocclusion based on current studies on consciousness and motor control may be the following. McFadden [85] suggested that conscious awareness or perception is a product of the brain’s electromagnetic field. Perception correlates with the synchronous firing of cortical neurons, thus generating the conscious electromagnetic field, which initiates conscious learning of motor activities. In this view, the reduced sensory input of the periodontal receptors of the incisor teeth may have resulted in localized sensory deprivation of the cerebral cortex and hence in at least localized sensory integration dysfunction. This in turn prevented the massive depolarization and synchronous firing of cortical neurons required for the generation of the conscious electromagnetic field, resulting in impairment of perception and motor behavior of the mouth, especially of the skilled coordinated movements of chewing.
However, the closure of the open bite and the establishment of normal occlusion of the teeth, through the orthodontic treatment, resulted in the normal sensibility of the periodontal mechanoreceptors of the incisor teeth and their regular input processing of information in the cortex, followed by a normal sensory integration function, a key to normal sensation, perception, learning, memory, and motor control of muscles. Thus, the normal cortical sensorimotor integration function resulted in the perception of oral structures, as the patient’s awareness of the presence of the full complement of teeth in the mouth clearly suggests. The conscious perception of the oral structures was associated with the generation of the electromagnetic field in the brain, which initiated the relearning of the motor functions of the mouth and the improvement in oral neuromuscular function.
In neurological terms, the changes in oral sensation and perception of the mouth following the reconstruction of the occlusion of teeth by the orthodontic therapy, could hypothetically have been effected through the brain’s neuroplasticity. Neuroplasticity implies chemical and structural modifications in dendritic spines in response to learning of new experiences [3–5]. Morphological changes in the dendritic spine are key to its plasticity and processing [6, 7]. Conversely, electrical stimulation leading to long-term potentiation (LTP), leads to synaptogenesis, i.e., alteration in the number of spines by the formation of new ones [8, 41]. Spines on dendrites are considered as the major sites of presynaptic excitatory inputs of the brain [3, 15].
Thus, the orthodontic procedures might have stimulated the oral mechanosensory systems so that afferent nerves carrying action potentials from periodontal periosteum, muscles, tendons, oral mucosa, receptors, etc, converged to the dendritic spines of the cortical neurons, where they were pooled and integrated in the microtubules of the subsynaptic zone lying beneath the spinous synapses. The subsynaptic zone of dendrites gives rise to spines and it is the physical substrate of memory storage of new learned experiences [15, 38]. The resulting new frequencies of muscle information contraction patterns changed the oral motor behavior [85]. Bone reorganization in turn, was “tuned” to the new frequencies of muscles through bone mechanosensation and intraosseous production of electric fields traveling in bone cells connected by gap junctions (electrical synapses) [91].
In addition, the awareness of the presence of the full complement of teeth in the patient’s mouth suggests that the perceptual scheme of the teeth has been inscribed in memory. Bliss and Lomo [17] discovered that in the hippocampus memory center there are special neuronal circuits serving long-term memory (LTP), and that brief experiences can induce memory storage lasting for a long time after the stimulus has been stopped.
In sum, the “orthodontic stimulus” has induced learning of new sensory-perceptual and motor functions of the mouth through the transformations of the corresponding sensory-perceptual, motor and memory areas of the brain. This might imply that the orthodontic stimulus has activated specific brain genes expressing plasticity. This might in turn imply that the rules that govern the structure and function of sensory and motor maps in the cortex can be decoded by the orthodontic stimulus, suggesting that the formation of cortical maps is experience-driven.
This insight is emancipating, because most molecular biologists were certain that environmental factors, such as tactile and pressure experiences could not affect gene function [69, 73]. Schanberg [73], however, located a growth gene that is responsible for the relationship between touch stimulation and growth. Schanberg found that touch deprivation of rat pups by their mothers resulted in growth deprivation through endocrine hormone effects. He then suggested that the brain can regulate growth genes through sensory (touch) processes affecting the production of growth hormone. Similarly, Moss [91] suggested that mechanotransduction of stimuli and computational bone biology offers an explanatory chain extending from the epigenetic event of muscle contraction hierarchically downward to the regulation of the bone cell genome.
3.4 Sensory Deprivation and Occlusal Interferences
The presence of irregularities of the teeth with premature contacts during closure, has long been considered to be a key factor in oral neuromuscular dysfunction, irrespective of whether it is assumed to operate by central initiation of reflex hyperactivity or by causing local hyperactivity of muscles at the brainstem level [95]. However, although many patients with this dysfunction do possess a defective occlusion, so too do a similar proportion of the population without this dysfunction [96]. This means that there is not a direct cause and effect relationship between occlusion pattern and dysfunction [95].
In the following paragraphs an attempt is made to relate occlusal interferences or premature dental contacts and the associated neuromuscular dysfunction, to sensory deprivation of the cerebral cortex. Sensory deprivation causes breakdown of the relative balance between the function of the ARAS and of cortical activity. Consequently, the individual becomes more alert, and this stage of the brain tends to facilitate the irrelevant stimuli, resulting in hyperactivity of muscles [64].
Recent neurophysiological studies of forces applied to the crown of teeth (tooth loads are essential for the fine motor control of the jaw muscles during mastication) have indicated that the periodontal mechanoreceptors are directionally sensitive to tooth loading. For example, the receptors of the incisor teeth and premolars are most sensitive (discharge vigorously) to forces applied horizontally to their crowns. This means that forces of the same magnitude applied in other directions do not evoke as great a response as the horizontal forces. This implies that fewer receptors respond in non-horizontal forces, providing ambiguous information to the central nervous system about the amplitude of the force [97, 98].
In addition, other neurophysiological studies have indicated that the periodontal mechanoreceptors signal only relatively small forces and saturate when larger forces are applied to the teeth, resulting in no useful information to the brain as to their magnitude. Thus, the sustained force level below which the mechanoreceptors signal is about 1 N (corresponding to 98 g weight) for the anterior teeth and about 3–4 N for the posterior teeth [99].
Accordingly, when a force of, for instance, 1 N is applied to an anterior tooth, the tooth moves slightly in the socket. Clinically, this sometimes is observed as fremitus which induces stress and strain in the periodontal ligament. A depolarizing receptor potential and an action potential are generated in the receptors and in the nerve terminals, respectively. The output then from an individual periodontal mechanoreceptor depends on the effectiveness of the fremitus in producing strain in the periodontal receptors, as well as of the directional sensitivity of the receptors to generate a vigorous action potential in the nerve terminals [93].
Thus a large number of mechanical factors determine the effectiveness of the movement of a tooth producing strain in the periodontal ligament and stimulation of receptors. These factors include the size of the force, the size and shape of the tooth, the point of rotation (fulcrum) of the tooth, the point of application of the force, and the presence of adjacent teeth with proximal contacts. The effect of these factors on the receptor depends on the precise location of the receptors about the root, the receptors’ orientation with respect to the surrounding collagen fibers under tension, and the viscoelastic properties of the periodontal ligament [93]. As a consequence of these factors each receptor is optimally stimulated by forces applied in directions that most effectively strain the receptors (directional sensitivity) [93].
It is also noted that in the normal dentition the receptive field of the periodontal mechanoreceptors extends beyond a single tooth to adjacent teeth through the interdental contact and the transeptal collagen fibers, resulting in a multitooth receptive field response [100]. The receptive field, however, might be even greater considering the fact that the bone cells (except the osteoclasts) are connected by gap junctions thus forming large cellular networks (syncytium), so that the electric fields generated by the physical loading of the alveolar bone are “tunneling” through the gap junctions into the entire bone cellular network [91]. In malocclusions of the teeth, however, only the “high spots” are in contact during closure. The proximal contacts are lost, because the teeth involved in the premature contacts are hypererupted. Consequently, the multitooth receptive field response might be considerably reduced, resulting in very little sensory input to the brain.
Also, there might be an additional reason contributing to the reduction in the sensory input, as follows. The force exerted on a hypererupted tooth during chewing or closure of the teeth might be higher than the level of loading the receptors can tolerate. For instance, if the force that is applied on a high spot in the anterior segment of the dentition is higher than 1 N, then its periodontal receptors saturate and provide no useful information to the brain [99]. Accordingly, the cerebral cortex may experience localized sensory deprivation, considering the large innervation and receptive field of the anterior teeth, resulting in neuromuscular dysfunction.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


