Biomaterial
Source
Advantages
Limitations
Natural polymers
Polysaccharides
Alginate
Dextran
Chitosan
Cellulose
Starch
Hyaluronan
Plant, animal
Sea algae
Bacteria
Crustaceans
Plant cell walls
Crops
ECM
Derived from renewable sources
Large diversity
Unique but complex structures
Functional groups, tailorable chemistry
Specific recognition domains
Intrinsic biodegradability
Composition of hybrid materials
Number of biologically derived polymers is limited
Difficult to process
Properties may differ
Undesirable immunoresponse
Pathogen transmission
Extracellular matrix
Tissue specific
Temporary-controlled growth factor release
Difficult to process and sterilize
Favorable environment for constructive tissue remodeling
Batch variations
Processing into many forms possible
Synthetic polymers
Polyester
PLA: poly(lactic) acid
PGA: poly(glycolic) acid
PLGA: copolymer of PLA and PGA
PCL: poly(ε-caprolactone)
Poly(urethanes)
Poly(ether ester)
PEG: poly(ethylene glycol)
PBT: poly(butylene terephthalate)
Minimal foreign body reaction
Tailorable mechanical properties and degradation rate, wettability, and protein adsorption
Degradation products present in the metabolic pathways
Functional groups to attract cells or bind growth factors
Cheap and reproducible production
Easily processed into any shape
Environment unlike ECM
Accumulation of acidic degradation products
Hydrogels
Natural materials
Collagen
Fibrin
Proteoglycans, HA
Chitosan
Alginate
Synthetic materials
PEG: poly(ethylene glycol)
SAP: self-assembling peptides
High biocompatibility
Tissue-like water content
Viscoelastic properties similar to soft connective tissue
Efficient transport of nutrients and waste
Uniform cell encapsulation
Injection and gelation in situ
Chemical or physical cross-linking
Modifications with biofunctional molecules or growth factors possible
Disadvantages of the individual material source
Due to low mechanical stiffness not suitable for certain applications, e.g., bone grafts
Bioceramics
Calcium phosphates
Biocompatible
Brittle
Hydroxyapatite
Excellent bone-bonding properties
Subject to fatigue
Tricalcium phosphate
Biodegradable
Decrease of mechanical strength under humid conditions
Biphasic calcium phosphate (HA/TCP)
Osteoconductive
Bioactive glasses
Variable rates of degradation
Silica-based glasses
18.3 Requirements for Scaffold Materials
For most regenerative strategies, an organic scaffold is used which should facilitate the attachment, migration, proliferation, and three-dimensional spatial organization of the cell population required for structural and functional replacement of the target tissue. As the science of tissue engineering is progressing, the definition of an ideal scaffold material is still unclear due to complex considerations which involve scaffold architecture and geometry, structural mechanics, surface properties, degradation characteristics, and composition of biological components as well as the change of these factors in in vitro or in vivo systems over time. It is unlikely that a single scaffold could serve as a universal foundation for the regeneration of different tissues. However, general requirements regarding physical, chemical, and biochemical properties can be defined. In either case, a scaffold material has to be nontoxic, biocompatible, and non-immunogenic to avert damage to neighboring cells and prevent adverse tissue reactions. Biodegradability by enzymes or hydrolysis is desired if the scaffold serves as a temporary template to provide support for the transplanted cells, and the degradation rate should match the cells’ rate of extracellular matrix production to enable constructive remodeling. This is characterized by scaffold degradation, cellular infiltration, vascularization, differentiation and spatial organization of the cells, and eventually replacement of the scaffold by the appropriate tissues. As carriers for drugs and differentiation factors, the materials should be versatile enough to enable the incorporation and controlled release of bioactive molecules.
Furthermore, any scaffold material must provide sufficient mechanical strength to substitute for the mechanical function of the diseased or damaged tissue that should be regenerated. The three-dimensional scaffold needs to maintain sufficient mechanical support to the transplanted cells during the growth and remodeling process, where the degree of remodeling depends on the cell number and cell type as well as on the tissue itself. The scaffold architecture and chemistry have to enable initial cell attachment, subsequent migration into or through the scaffold, transfer of nutrients and metabolites, as well as provision of sufficient space for the development and later reconstruction of the organized tissue. From a clinical point of view, it must be possible to fabricate the scaffold material under GMP (good manufacturing practice) conditions in a quality-controlled and reproducible fashion.
In one scenario, cells can be seeded onto the scaffold and cultured in vitro to generate the desired tissue before transplantation. A different approach is the design of materials for transplantation of a primarily cell-free system, which will, due to a combination of signaling molecules incorporated in the scaffold, induce the homing of stem cells residing in the respective tissues and promote their differentiation to support regeneration. Cell-free scaffolds are especially attractive because of an easier handling process that eliminates the issues associated with the use of stem cells and their expansion in vitro, with storage and shelf life, cost aspects, immunoresponse of the host, and transmission of diseases [27].
18.4 Dental Pulp Tissue Engineering
Although dentistry is one of the disciplines which have capitalized on the use of biomaterials for the longest time, these have served mainly to replace lost tissues and restore esthetics and function. However, tissue engineering approaches in dentistry are evolving. In the fields of periodontology and oral surgery, regenerative strategies have already been implemented in daily practice. Commercially available products for bone and periodontal tissue regeneration are available to clinicians and have improved treatment outcomes and success rates [28]. More recently, engineering of dental pulp and dentin using pulp-derived stem cells has made considerable progress.
The fact that dental pulp possesses regenerative capabilities has been known for several decades. Stimuli such as bacterial toxins or tissue damage will lead to an upregulation of odontoblast activity, and these cells will produce a reparative dentin as an active defense mechanism to separate the soft tissue from the site of injury. Even after exposure of pulp tissue in deep cavities and disruption of the odontoblast layer, regeneration is possible after application of medicaments such as calcium hydroxide or mineral trioxide aggregate (MTA), which disinfect due to a high pH, cause necrosis in the adjacent cell layer, and stimulate defense mechanisms and reparative dentin formation [29, 30]. However, pulp’s capacity to regenerate is limited, unless we find ways to more effectively exploit its intrinsic healing potential. If regeneration fails and inflammation persists, non-regenerative endodontic treatment can keep the tooth functional but terminates dentin formation and root maturation and will leave the tooth deprived of its soft tissue (devital) and weakened.
Several groups have begun to develop strategies to engineer dental pulp. The two categories of materials that are most commonly used in tissue engineering are synthetic polymers such as PLA and PGA [31] and matrices derived from biological sources such as reconstituted collagen [10]. Table 18.2 provides an overview of biomaterials that have been utilized particularly for dental pulp tissue engineering.
Table 18.2
Biomaterials that have been utilized particularly for dental pulp tissue engineering
|
Material
|
Engineering approach
|
Result
|
Reference
|
|---|---|---|---|
|
PGA, collagen I, alginate
|
Pulp fibroblasts seeded onto different materials, cell culture in vitro
|
Pulp-like tissue after 45–60 days on PGA
|
Mooney et al. [32]
Bohl et al. [33]
|
|
HA/TCP
|
Stem cells from dental pulp (SHED, DPSC) mixed with HA/TCP powder transplanted into nude mice
|
Generation of dentin or bone (SHED) and dentin–pulp-like complexes (DPSC)
|
Gronthos et al. [1]
Miura et al. [2]
|
|
Collagen I and III, chitosan, gelatin
|
Human dental pulp cells seeded into different materials for comparison in vitro
|
Adhesion and proliferation:
|
Kim et al. [100]
|
|
Col I > Col III > gelatin> > chitosan
|
|||
|
ALP activity:
|
|||
|
Col I > Col III > gelatin> > chitosan
|
|||
|
Mineralization: Col I > Col III > gelatin
|
|||
|
Oc, Dspp, and Dmp-1 expression on collagen
|
|||
|
Collagen I with Dmp-1
|
Collagen scaffolds laden with Dmp-1 and dental pulp stem cells were placed in dentin disks with a simulated furcal perforation and transplanted subcutaneously into nude mice
|
Formation and organization of new pulp tissue
|
Prescott et al. [3]
|
|
PLA
|
SHED seeded onto PLA scaffolds into tooth slices, subcutaneous transplantation into nude mice
|
Formation of vascularized soft connective, pulp-like tissue and new tubular dentin
|
Cordeiro et al. [6]
Sakai et al. [7]
|
|
PLGA
|
SCAP and DPSC seeded onto PLGA into root canals sealed with MTA on one side, subcutaneous implantation into nude mice for 3–4 months
|
Formation of a pulp-like tissue, deposition of dentin along the root canal wall
|
Huang et al. [34]
|
In 1996, a pulp-like tissue was first engineered in vitro after seeding pulp fibroblasts on PGA fibers, where cells formed new tissue after 60 days in culture [32]. Using a similar approach, the ability of different scaffold materials to support pulp tissue formation from pulp fibroblasts was evaluated 2 years later. PGA, collagen hydrogels, and alginate were tested, where culturing of cells on PGA resulted in tissue formation and collagen synthesis, whereas only moderate cell proliferation was observed on the collagen scaffold and no proliferation on alginate [33].
Recently, formation of pulpal tissue could be demonstrated in vivo. After isolation and in vitro characterization of dental pulp stem cells from deciduous teeth and third molars, these cells were mixed with hydroxyapatite/tricalcium phosphate (HA/TCP), and the formation of dentin, bone, and dentin–pulp-like complexes was observed [1, 2]. Highly promising outcomes have been reported by Nör’s group, where dental stem cells were seeded on PLA scaffolds and inserted into tooth slices after removal of the original pulp tissue. These constructs were implanted subcutaneously into immunodeficient mice. Formation of a vascularized pulp-like tissue with odontoblast-like cells and newly generated dentin was shown [6, 7]. Mobilization and release of growth factors and proteins from the dentin due to a locally decreased pH by degradation of PLA scaffolds might promote the differentiation process in this case [7]. Similarly, Huang et al. observed soft tissue and deposition of new dentin after transplantation of stem cells from apical papilla (SCAP) on PGLA into an empty root canal space [34]. In previous own work, dental pulp stem cells were seeded into self-assembling peptide hydrogels together with growth factors TGFβ1, FGF-2, and VEGF. The cell–gel mixture was injected into dentin cylinders prepared from extracted human teeth and transplanted subcutaneously into immunocompromised mice. After 5 weeks in situ, a vascularized pulp-like tissue had formed within the dentin cylinders, the cells lining the dentin expressed dentin sialoprotein as an indicator of differentiated odontoblast-like cells, and these cells extended processes into the dentinal tubules similar to the physiological situation [35].
Nakashima et al. developed a pulpotomy model in canines, where the pulp chamber was accessed, the coronal pulp was removed, and the pulp chamber was filled with an angiogenic subpopulation of dental pulp stem cells on a collagen type I carrier laden with the growth factor SDF-1 (stromal-derived factor 1). After 6 weeks, the pulp chamber was filled with pulp-like tissue which could not be distinguished from the original pulp, it was vascularized, and tubular dentin formation had taken place [4]. Taking it one step further, the group extracted teeth in dogs and replanted them after drilling an access cavity to the pulp chamber, pulpectomy and apexectomy, and filling of the root canal with a collagen scaffold, stem cells, and SDF-1. A pulp-like tissue could be found along the root canal, showing that after transplantation of stem cells, dental pulp tissue engineering was possible [5].
In summary, collagen I and the synthetic polymers showed the most favorable results among the materials studied for this particular application. In terms of biocompatibility and degradation, all the previously described materials exhibit satisfactory results. Synthetic polyester such as PLA, PGA, and their copolymers are nontoxic and biocompatible; they degrade by hydrolysis and have gained FDA approval for various applications [17]. Collagen is biocompatible and degradable by enzymes, but natural polymers are often difficult to process and to modify and generally afflicted with the risk of transmitting animal-associated pathogens or provoking an immunoresponse. Alginate, a polysaccharide derived from red algae, offers a mild cell encapsulation process as it can be cross-linked via Ca2+. However, it degrades in a rather uncontrolled manner via dissolution, as the material is sensitive to calcium chelating compounds [36]. Chitosan is derived from chitin, a polysaccharide found in crustaceans. Due to its biocompatibility and degradability via naturally occurring enzymes, it has been used for numerous tissue engineering applications [37].
18.5 Growth and Differentiation Factors
Many signaling pathways and molecules are similar during tooth development and tooth reparative responses. Increased knowledge of the biological cues mediating these processes enables investigators to mimic or supplement regeneration or repair of dental tissues.
Growth factors, especially those of the transforming growth factor-beta (TGFb) family, are important in cellular signaling for proliferation, differentiation, and induction of odontoblast secretory activity. These growth factors are secreted by dentin-forming cells during tooth development and correlate with specific events regarding morphogenesis, histogenesis, and cytodifferentiation [38, 39]. Another important family of growth factors in tooth development and regeneration is the bone morphogenetic proteins (BMPs). Recombinant human BMP-2 stimulates differentiation of postnatal pulp stem cells into odontoblast-like cells in culture [40, 41]. In addition, recombinant BMP-2, BMP-4, and BMP-7 have been shown to induce formation of reparative dentin in vivo [42, 43]. Apart from growth factors, other molecules have been demonstrated to induce pulp cell differentiation. Dentin matrix protein-1, a non-collagenous protein involved in the mineralization process, stimulated cytodifferentiation, collagen production, and deposition of calcified tissue in dental pulp in a rat model [44]. Dexamethasone, a synthetic glucocorticoid, reduced cell proliferation and stimulated the expression of mineralization-associated markers such as alkaline phosphatase and dentin sialophosphoprotein in primary human pulp cells [45]. Addition of β-glycerophosphate to explants from human teeth induced a change in cell morphology, collagen synthesis, and mineral formation [46]. Combinations of inorganic phosphate and dexamethasone have been used as standard osteogenic supplements to drive the differentiation of mesenchymal stem cells into osteoblasts as well odontoblast-like cells followed by mineral deposition [1, 2]. This may be explained by the fact that osteogenesis and dentinogenesis are similar processes, and bone-forming osteoblasts and dentin-forming odontoblasts are closely related cell lineages. However, they remain distinct cell types, as observed by their slightly different gene expression profile and the obvious structural differences of their respective products. Optimal conditions permissive for dental stem cell differentiation into odontoblasts rather than osteoblasts remain to be elucidated. The increasing knowledge about the underlying biological processes enables us to develop materials which allow for the incorporation of biological cues in order to stimulate a desired cellular response.
Whereas the addition of exogenous growth and differentiation factors is one way to drive cell proliferation, differentiation, and tissue formation, questions and problems regarding the optimum concentrations and the possibility of undesirable side effects including tumorogenesis remain. An alternative is the recruitment of endogenous growth factors, which are present in the dentin matrix itself. During tooth development, the odontoblasts secrete a variety of growth factors, neurotrophic factors, and cytokines which are deposited within the organic matrix preceding the mineralized tissue [47–52]. During mineralization, these bioactive factors become embedded and immobilized in the dentin matrix [47, 53]. Whereas proteins and growth factors in an active form have a short half-life, binding to extracellular matrix components may be required to maintain their bioactivity by protecting them from proteolytic degradation and thus prolonging their life span. Among growth-factor-binding compounds are proteoglycans, mainly heparan sulfate [53], furthermore specific binding proteins [54], glycoproteins such as fibronectin [55], or different types of collagen [56, 57].
As there is no turnover in dentin extracellular matrix, bioactive regulatory molecules can be reactivated much later in life upon release from their bond. Organic acids or chelating agents such as EDTA (ethylenediaminetetraacetic acid) are suitable for dentin demineralization. EDTA acts as a potent chelator which binds and withdraws calcium ions from solution and thus alters the balance between binding and release of ions of the hydroxyapatite crystal lattice. A variety of growth factors have been shown to be present in the EDTA-soluble fraction of demineralized human dentin extracellular matrix, including transforming growth factor β1 (TGF-β1), fibroblast growth factor 2 (FGF-2), bone morphogenetic protein 2 (BMP-2), platelet-derived growth factor (PDGF), placenta growth factor (PIGF), epidermal growth factor (EGF), but also angiogenic factors such as vascular endothelial growth factor (VEGF) [47–52]. These molecules are effective at very low concentrations and elicit cellular responses still at picogram concentrations, modifying immunodefense, angiogenesis, cell recruitment, proliferation, and differentiation as well as mineralization [58–61].
As described previously, growth factors in natural extracellular matrix are bound to ECM elements. This feature can be mimicked by synthetic matrices, for example, via binding to heparin. This negatively charged glycosaminoglycan can be incorporated into scaffold materials and in turn bind to growth factors such as TGF-β1, FGF-2, or VEGF. Binding and slow release of these growth factors from synthetic scaffolding materials has been demonstrated and can be taken advantage of [62, 63].
18.6 Cell-Based vs. Cell-Free Approaches to Dental Pulp Engineering
It is accepted to date that after transplantation of stem cells into the root canal, dental pulp tissue engineering is possible [4–7, 34, 35]. If the goal is to develop a clinical procedure of stem cell transplantation for pulp regeneration, the choice of scaffold material seems to be of secondary importance, as we can utilize the stem cells’ inherent competence to form pulp tissue, especially in contact with the appropriate matrix, the dentin. However, cell-based tissue engineering of dental pulp is afflicted with several problems. At the time point and age when dental stem cells are available from a patient (loss of primary teeth, removal of wisdom teeth), therapies to regenerate dental pulp are usually not necessary yet. Thus, stem cells need to be stored, but banking of stem cells as well as expansion in culture before transplantation is costly. Even if money issues do not matter, it can barely be envisioned to perform a transplantation procedure in a dental office but has to be accomplished in a hospital where GMP guidelines are followed. Generally, it appears that the benefit does not outweigh the cost and effort.
A different approach to pulp regeneration is cell-free regeneration or cell homing. In that case, a scaffold material in combination with growth factors is supposed to recruit resident stem cells and attract them to populate the scaffold material, proliferate, differentiate, and form a three-dimensional tissue [27]. In that case, cells could be recruited either from remnant vital pulp tissue after pulpotomy or, if the pulp tissue is lost, from the periapical region. In the case of cell homing, the scaffold material plays a crucial role and should be tailor-made for this specific application. The envisioned clinical procedure for dental pulp regeneration using stem cell homing is depicted in Fig. 18.1.
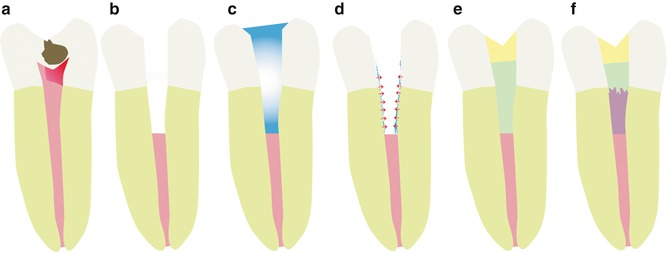

Fig. 18.1
Clinical procedure for dental pulp regeneration using stem cell homing. (a) Irreversible pulpitis. (b) Pulpotomy. (c) EDTA conditioning. (d) Release of growth factors. (e) Insertion of biomaterial. (f) Regeneration (Used with permission of Elsevier. Galler et al. [99])
Ideally, the scaffold material should most closely resemble the cells’ physiological environment – natural extracellular matrix. The ECM acts as a structural support, but its role goes far beyond this. It is a nanostructured environment that provides the biochemical cues to modulate cellular behavior and reinforce a particular phenotype. Furthermore, the ECM is dynamic; it can be selectively degraded and remodeled by the cells living within it. Polymers like PLA have the advantage of being biodegradable, biocompatible, inexpensive, and easy to prepare. However, they lack the chemical information that can be found in the ECM physiologically. On the other hand, collagen offers the chemical and structural information of the ECM but is difficult to customize for specific applications. Because of its biological origin, purity and immune reaction can be an issue. An ideal scaffold should combine the best properties of each of these groups of biomaterials. These would be structurally similar to ECM at the nanoscale, be able to present complex molecular information to the cells, and be easy to modify for specific applications. To address these deficiencies novel synthetic matrices are developed for tissue engineering. Among these, peptide-based nanofibers are an example of a tunable, ECM-like matrix and are particularly promising due to their ease of synthesis, chemical diversity, and high control over various aspects of material behavior [25, 62, 64–66]. Regarding dentin–pulp-complex engineering, the scaffold should allow us to address the particular challenges of this approach, including injectability into the root canal, contamination control, vascularization and innervation of a long and narrow space, the incorporation of growth and differentiation factors relevant to odontoblast differentiation, the support of mineral formation, and the possibility to create and insert primarily cell-free matrices, which are capable of recruiting resident stem cells in the respective tissues.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


