A sarcoma is a malignant neoplasm originating from mesenchyme, or embryonic connective tissue. Carcinomas are significantly more common than sarcomas in both bone and soft tissue; however, sarcomas of bone are important lesions because they require extensive management and have a serious prognosis.
This chapter discusses osteosarcoma, chondrosarcoma, radiation-induced sarcoma, and Ewing’s sarcoma. Multiple myeloma is of hematopoietic origin and is discussed because it is more commonly encountered than the sarcomas just mentioned. Metastatic carcinoma to the jaws is mentioned in this chapter because it is, by far, the most common malignant tumor of the jaws. The chapter discusses epidemiologic, clinical, radiographic, and histopathologic features, as well as treatment and prognosis. An extensive section covering surgical management of patients with malignant tumors of the jaws is included.
OSTEOSARCOMA (OSTEOGENIC SARCOMA)
Osteosarcoma is a tumor composed of malignant connective tissue cells directly producing osteoid and bone, and in some cases cartilage.
CLINICAL AND RADIOGRAPHIC FEATURES
Osteosarcoma is the most common primary malignancy of bone after multiple myeloma, and it makes up about 20% of all sarcomas of the skeleton. About 900 patients are newly diagnosed with osteosarcoma each year in the United States. Osteosarcoma arises most frequently in the femur, tibia, and humerus. About half of all cases develop in the region of the knee. It occurs throughout life but is most common in the second decade.
Approximately 5% to 7% of all osteosarcomas occur in the jaws. The peak incidence of jaw lesions is in the fourth decade, with a mean of 34 years. This is one to two decades later than the peak incidence in the extragnathic skeleton. Cases have been reported in patients of all ages.
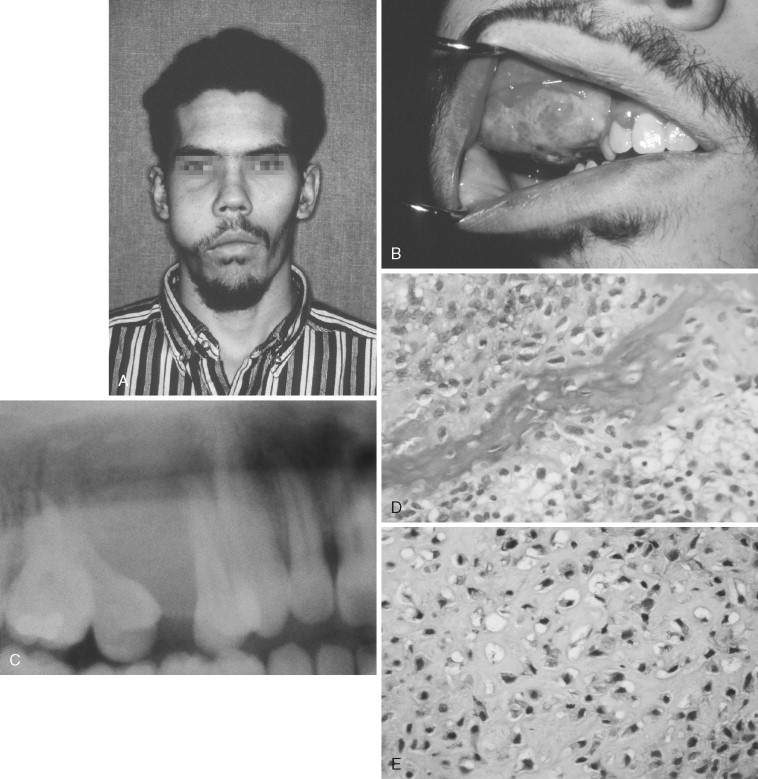
Osteosarcoma of the jaws most commonly presents with swelling and localized pain. Note, however, that pain is not a constant presenting complaint, as shown in a study of 30 patients whose most common presenting symptom was swelling without pain. The lesions in 13 patients in this study were initially misdiagnosed as odontogenic infections.
Other signs and symptoms include loosening and displacement of teeth, paresthesia, epistaxis, and nasal obstruction. The average duration of symptoms before diagnosis is 3 to 4 months. The most common locations are the body of the mandible and alveolar ridge of the maxilla.
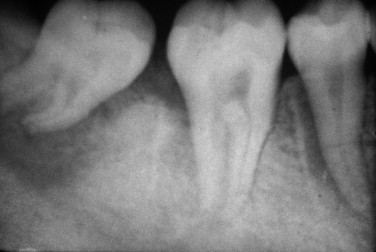
The radiographic appearance of osteosarcoma of the jaws depends upon the interaction of three factors: destruction of bone, osteoid production and its mineralization, and new bone formation by the periosteum. Lesions can thus appear entirely radiolucent; radiolucent with fluffy, cloudlike radiopaque areas; or entirely radiopaque ( Figures 34-1 and 34-2 ). The majority of tumors (60% to 80%) produce a mixed radiolucent-radiopaque pattern with poorly defined, irregular borders. The tumor commonly perforates the cortex and extends into soft tissue.
A “sunray” or “sunburst” radiographic pattern has been classically described for osteosarcoma. It results when the periosteum produces spicules of bone perpendicular to its surface. This pattern can occur in the jaws and is best visualized by an occlusal radiograph. A number of authors have emphasized that the sunray pattern is not present in all osteosarcomas and, when present, is not diagnostic of osteosarcoma. It can be seen with neoplastic and reactive lesions that cause a periosteal reaction. The same comments apply to the “onionskin” periosteal reaction sometimes present in osteosarcoma.
Early osteosarcoma of the jaws may demonstrate subtle radiographic changes. An important early radiographic feature is symmetric widening of the periodontal ligament space around teeth in the area of the lesion as a result of infiltration of tumor into the periodontal ligament ( Figure 34-2 ). This feature is not specific for osteosarcoma but explains why early osteosarcoma is often misdiagnosed as odontogenic inflammatory disease, because both are characterized by localized pain and widening of the periodontal ligament space. Destruction of the lamina dura and resorption of involved tooth roots may also occur.
In a study of 46 craniofacial osteosarcomas, including 24 of the jaws, the authors noted that conventional plain radiographs are of limited value because of superimposed bony structures, although these radiographs can be useful adjuncts when the patient has extensive dental restorations. Computed tomography (CT) produces excellent images of tumor calcification, involvement of the cortical bone, and extension of tumor into soft tissue and medullary spaces. Magnetic resonance imaging (MRI) is even better for detecting tumor in soft tissue.
METASTASIS
Osteosarcoma of the skeleton metastasizes most commonly by the hematogenous route rather than by lymphatic vessels. At the time of diagnosis, clinically apparent metastatic disease is present in 10% to 20% of all patients. At autopsy the most common sites of metastasis are the lungs, bones, and kidneys. Osteosarcoma of the jaws does not metastasize as often as extragnathic lesions. The most common sites are lung and brain.
HISTOPATHOLOGIC FEATURES
The microscopic diagnosis of osteosarcoma depends upon finding malignant connective tissue stroma (malignant osteoblasts) that directly produces osteoid and bone. The malignant osteoblasts demonstrate pleomorphic and hyperchromatic nuclei and bizarre mitoses ( Figure 34-3 ). Malignant cartilage may also be present and in some lesions may be the predominant component ( Figure 34-1, E ). However, the tumor is diagnosed as osteosarcoma if malignant osteoid or bone is identified. Areas of necrosis, fibrosis and calcification, and multinucleated giant cells may also be present.
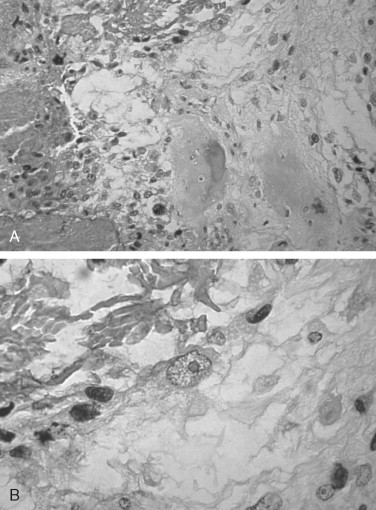
Osteosarcoma has been divided into three histologic subtypes based on the dominant cell type or component: fibroblastic, chondroblastic, or osteoblastic. These histologic subtypes do not appear to be associated with the clinical behavior or prognosis of the lesion. Jaw lesions tend to be predominantly chondroblastic.
Small-cell osteosarcoma is a histologic subtype that does have prognostic significance. It is composed of small round cells somewhat similar to those seen in Ewing’s sarcoma, but the malignant osteoblasts produce osteoid. Small-cell osteosarcoma constitutes 1% to 4% of all osteosarcomas. It has a poor prognosis, worse than that of conventional osteosarcoma, regardless of treatment.
Low-grade osteosarcoma is a histologic variant that makes up less than 2% of all osteosarcomas occurring in the skeleton. It arises within the medullary cavity of bone and only rarely occurs in the jaws. Its importance lies in the fact that because it is histologically well-differentiated it is commonly misdiagnosed as a benign lesion, particularly when the biopsy specimen is small. The tumor demonstrates minimal pleomorphism and mitoses and has been misdiagnosed as fibrous dysplasia. The microscopic features of low-grade osteosarcoma that distinguish it from benign lesions include destruction of cortical bone, possession of poorly defined margins, and invasion of soft tissue. Even though the lesion is histologically low-grade it is treated the same as conventional osteosarcoma—complete excision with clear margins. The prognosis is good.
Other benign lesions that can be confused microscopically with osteosarcoma include a callus of a healing fracture, osteoblastoma, cementoblastoma, and active or aggressive ossifying fibroma. The histopathologic features must be correlated with clinical and radiographic information to arrive at a diagnosis.
SECONDARY OSTEOSARCOMA
In patients older than 60 years, bone sarcomas are more frequently secondary to other bone conditions. Osteosarcoma has been reported to arise secondary to, or in association with, Paget’s disease of bone, radiation therapy to the region, fibrous dysplasia, bone infarcts, chronic osteomyelitis, and familial retinoblastoma. Radiation-induced sarcoma is discussed later.
The incidence of malignant transformation in patients with Paget’s disease of bone has been reported to be 0.7%. The most frequent tumor was osteosarcoma, followed by malignant fibrous histiocytoma, fibrosarcoma, and chondrosarcoma. It is estimated that 3% to 14% of all osteosarcomas of bone arise in patients with Paget’s disease. The prognosis for these tumors is poor, with 69% of patients dying within 2 years after diagnosis.
MOLECULAR GENETICS OF OSTEOSARCOMA
Alterations in several genes have been associated with osteosarcoma. Some of the genes that appear to play a role in the development of osteosarcoma include MDM2 (murine double minute 2), CDK4 (cyclin-dependent kinase 4), and SAS (sarcoma amplified sequence). Mutations and/or overexpression of these genes results in altered protein expression with loss of control of cell proliferation and differentiation.
TREATMENT AND PROGNOSIS OF CONVENTIONAL OSTEOSARCOMA
Before the advent of adjuvant chemotherapy, the treatment of choice for osteosarcoma was radical surgical excision, with 5-year survival rates for lesions in long bones reported as 20% to 30%. In 1993 the Cooperative Osteosarcoma Study Group reported on the use of high-dose methotrexate, doxorubicin, and cisplatin (BCD) for adjuvant chemotherapy in osteosarcoma of the extremities. They achieved 8-year metastasis-free survival rates of 60% to 70%. One of the most important prognostic factors is tumor response to chemotherapy. Patients with tumors that respond with greater than 90% tumor necrosis after chemotherapy have a significantly better prognosis. Radiation therapy appears to have no significant benefit.
As mentioned previously, the biologic behavior of osteosarcoma of the jaws is significantly different than its behavior in the extragnathic skeleton; specifically, metastasis is much more common in extragnathic sites, and death resulting from local recurrence is more common in lesions primary to the jaws. The 5-year survival rate for osteosarcoma of the jaws treated with radical surgery alone has been reported as 25% to 50% and many deaths were the result of uncontrolled local disease. It is important to note that complete surgical extirpation of the lesion with clear surgical margins is the cornerstone of treatment of all osteosarcomas of the jaws. Some authors report that the use of radical surgery plus multiple drug chemotherapy for treatment of osteosarcoma of the jaw appears to improve patients’ overall prognosis and disease-free survival rates. Other studies report no improvement in prognosis of jaw osteosarcoma with the addition of chemotherapy. Other authors believe that the influence of multiagent chemotherapy on prognosis of jaw lesions cannot be determined at this time because no randomized, carefully-controlled clinical studies have been conducted and future studies will be difficult because of the rare nature of this tumor.
A study of 30 patients with osteosarcoma of the jaws treated with combinations of surgery, radiation therapy, and chemotherapy revealed that factors associated with statistically significant poorer prognosis included neural sensory alteration as a presenting symptom, increasing age of patients, and surgical margins of less than 5 mm. There was a trend toward better survival in patients receiving chemotherapy with four or more agents.
In summary, many medical centers treating osteosarcoma of the jaws use multiagent chemotherapy, in select patients, in addition to radical surgical resection. The question of whether to use chemotherapy is described in the literature as contentious; however, it is advised that chemotherapy at least be considered in addition to radical surgery.
SURFACE (JUXTACORTICAL) OSTEOSARCOMA
Parosteal and periosteal osteosarcoma are two variants of surface osteosarcoma that arise from the periosteum of bone and initially grow outward. In contrast, conventional osteosarcoma arises in the medullary portion of the bone. These variants are important for the oral and maxillofacial surgeon because they have clinical and histopathologic features and prognoses different from those of conventional osteosarcoma.
Some authors argue that the term juxtacortical should be dropped as it is often used synonymously with parosteal . Parosteal osteogenic sarcoma is a slow-growing, lobulated or cauliflower-like, often painful lesion attached to the outer surface of the cortex by a broad base. It is generally radiopaque, more so at the base than at the periphery of the lesion. The periosteum appears radiographically as a radiolucent space separating the tumor from the cortical plate.
Histopathologically, parosteal osteosarcoma is a low-grade (well-differentiated) tumor forming trabeculae of bone and foci of cartilage. The cells appear deceptively benign, demonstrating minimal atypia and mitotic activity. The microscopic differential diagnosis includes osteoma and osteochondroma. In the long bones parosteal lesions often have higher-grade malignant regions within them.
Periosteal osteosarcoma appears predominantly radiolucent, although it may demonstrate radiopaque spicules oriented perpendicularly to the periosteum. The cortex is thickened and has a scalloped surface. Histopathologically, the tumor is better differentiated than most conventional osteosarcomas, but less differentiated than parosteal tumors. It has a prominent cartilaginous component.
The treatment of parosteal and periosteal osteosarcomas should be radical surgical excision with tumor-free margins, as the lesions typically recur after curettage or local excision. Parosteal osteosarcoma has an excellent prognosis, with a 10-year survival rate greater than 80%. The presence of higher-grade areas may alter the treatment and prognosis. Periosteal osteosarcoma has a prognosis intermediate between those of parosteal and conventional osteosarcoma. *
* , , , , , .
CHONDROSARCOMA
Chondrosarcoma is a malignant tumor in which the tumor cells produce cartilage but not bone. It is notoriously difficult to distinguish microscopically between benign and malignant cartilaginous tumors. Garrington and Collett, in their extensive reviews on chondrosarcoma, state that “probably more than any other group of tumors, cartilage tumors are even today universally regarded with suspicion and distrust.”
CLASSIFICATION
Chondrosarcoma is designated as central or peripheral, depending upon whether it arises within bone or on its surface, respectively. Primary chondrosarcoma arises de novo, whereas secondary tumors originate in a preexisting benign chondroid lesion. Extraosseous chondrosarcoma arises in soft tissue outside the skeleton. Mesenchymal chondrosarcoma is a high-grade variant that has clinical and microscopic features distinct from those of conventional chondrosarcoma.
CLINICAL AND RADIOGRAPHIC FEATURES
Chondrosarcoma of the general skeleton comprises about 10% of all primary malignant bone tumors, making it about half as common as osteosarcoma. The peak ages of occurrence are between 30 and 50 years.
Chondrosarcoma of the jaws is a rare lesion. Although the peak incidence is at 20 to 40 years, many cases have been reported in old age. The most common chief complaint is a swelling or mass, with pain reported by less than half of patients at the time of initial diagnosis. Maxillary lesions may cause epistaxis or nasal obstruction. The most common locations are the anterior region of the maxilla and the posterior mandible. Dental complaints may cause the patient to seek treatment. These include loosening, resorption, and exfoliation of teeth, as well as movement of teeth and formation of diastemata.
The radiographic appearance of chondrosarcoma of the jaws is variable. It can be radiolucent, radiolucent with radiopaque areas, or diffusely radiopaque. It most commonly presents as a poorly defined radiolucent lesion with radiopaque foci resulting from mineralization or ossification of the cartilage matrix. It occasionally causes symmetric widening of the periodontal ligament space and a “sunburst” pattern. These features are similar to what has been described in osteosarcoma. Occasionally, a chondrosarcoma can infiltrate between trabeculae of normal bone without significantly destroying bone. The intact trabecular bone has an essentially normal radiographic appearance even though the tumor can be extensive.
HISTOPATHOLOGIC FEATURES
The microscopic features of chondrosarcoma vary from almost benign to frankly malignant. Some tumors have abundant cartilage matrix, whereas others have almost none. The neoplastic chondrocytes typically are arranged in nests or groups. The nuclei are variable in size and shape. Many chondrocytes are binucleated: that is, two nuclei are present within one lacuna ( Figure 34-4 ). The number of mitotic figures increases with the increasing grade of the tumor. The cartilage intercellular matrix varies from mature hyaline cartilage that can become calcified to myxoid stroma, which is connective tissue that contains an increased amount of ground substance that results in lightly basophilic staining. Bone may be seen in the tumor, but it is residual or newly formed normal bone made by the host as a reaction to the tumor. There is no bone directly formed by the tumor cells.
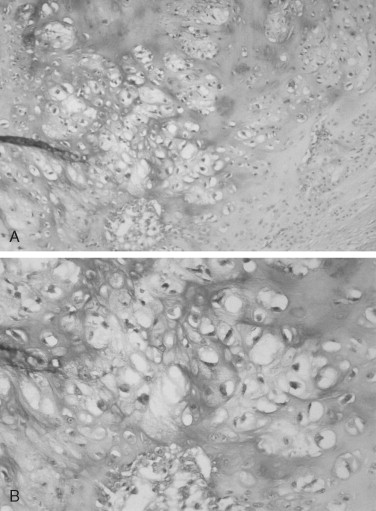
The grading system established for chondrosarcoma is important because it is related to prognosis. A three-grade system is used, with grade 1 the best differentiated. Grade 1 lesions have chondrocytes with nuclei that appear normal or slightly enlarged and occasionally binucleated. Mitotic figures are scarce. Grade 1 lesions cause the most difficulty in diagnosis because of their similarity to chondroma. Grade 2 chondrosarcomas have less matrix and more cells, especially at the periphery of the tumor. The chondrocytes are enlarged, and often there is more than one cell per lacuna. Grade 3 lesions have the greatest cellularity and the least amount of cartilage matrix. The chondrocytes are bizarre in shape, and nuclei are typically greatly enlarged and pleomorphic. Necrosis is present and may be prominent. Mitoses are more numerous in grade 3 tumors but still may be difficult to find. In Garrington and Collett’s review of 37 cases, 46% of the tumors were grade 1, 41% were grade 2, and only 14% were grade 3. Their patients with grade 1 and grade 2 chondrosarcomas had almost the same survival rate, but both groups did much better than patients with grade 3 tumors.
An important concept for oral and maxillofacial surgeons is that chondrosarcomas usually have a dominant microscopic grade but may also contain small foci that are of different grades. If one small incisional biopsy of a tumor is performed, the specimen may demonstrate features that are not representative of the entire lesion. Wide surgical sampling of large tumors is important to obtain the most accurate diagnosis.
Another critical issue is the distinction between chondrosarcoma and chondroma. Some authors do not accept a diagnosis of a benign tumor of cartilage in the jaws and recommend that all cartilaginous tumors of the craniofacial bones, even if microscopically benign, be widely excised. However, cartilage remnants of embryologic development occur in the jaws, most commonly in the anterior maxilla. They are not neoplasms and are benign. It might be safer to state that chondrosarcomas of the jaw are reportedly more common than chondromas. When a surgeon receives a histopathologic diagnosis of chondroma, it is prudent to request a second opinion.
TREATMENT AND PROGNOSIS
Chondrosarcomas of the jaws do not appear to be radiosensitive and have not responded well to chemotherapy. The recommended management is radical surgical excision, bearing in mind that they may extend well beyond the apparent clinical or radiographic margins. Metastasis is uncommon with jaw lesions, and when it does occur, the lung and bone are the most common locations. Radical neck dissection is probably not indicated.
The prognosis for chondrosarcoma of the jaws appears to be influenced by the histologic grade of the tumor and specific site of origin, with maxillary tumors having the worst prognosis and tumors of the anterior mandible having the best survival rate. As with osteosarcoma of the jaws, the prognosis of chondrosarcoma depends upon complete surgical removal of the lesion with clear surgical margins.
The prognosis for chondrosarcoma compared with that of osteosarcoma of the jaws is controversial. A 1995 report from the Mayo Clinic states that chondrosarcoma of the jaws and facial bones is a locally aggressive tumor with a better prognosis than osteosarcoma. Reported survival rate in chondrosarcoma patients was 68% at 5 years and 44% at 15 years. Death was the result of uncontrolled local recurrence rather than metastasis. Other authors report a much poorer prognosis for jaw chondrosarcoma, with a 5-year survival rate of 15% to 20%.
MESENCHYMAL CHONDROSARCOMA
Mesenchymal chondrosarcoma is a rare tumor with sufficiently unique clinical and microscopic features to warrant consideration as a lesion distinct from conventional chondrosarcoma. Up to one third of mesenchymal chondrosarcomas arise in extraskeletal soft tissue. Lesions originating within bone have a predilection for the maxilla, mandible, and ribs. Most patients with this tumor are between the ages of 10 and 30 years. The most common presenting complaints are pain and swelling. Radiographically, the lesion appears as a destructive radiolucent lesion that may contain stippled calcification.
Histopathologic examination of mesenchymal chondrosarcoma reveals two distinct patterns. Sheets of small, undifferentiated round or spindle cells alternate with areas of well-differentiated cartilage. An adequate biopsy sample is necessary to reveal the areas of cartilage, as the small cells may be confused with Ewing’s sarcoma.
The recommended treatment for mesenchymal chondrosarcoma is radical surgical excision. It is a highly malignant tumor, and it metastasizes more frequently than conventional chondrosarcoma. The prognosis is poor, with a 20% to 30% long-term rate of survival.
CHONDROSARCOMA
Chondrosarcoma is a malignant tumor in which the tumor cells produce cartilage but not bone. It is notoriously difficult to distinguish microscopically between benign and malignant cartilaginous tumors. Garrington and Collett, in their extensive reviews on chondrosarcoma, state that “probably more than any other group of tumors, cartilage tumors are even today universally regarded with suspicion and distrust.”
CLASSIFICATION
Chondrosarcoma is designated as central or peripheral, depending upon whether it arises within bone or on its surface, respectively. Primary chondrosarcoma arises de novo, whereas secondary tumors originate in a preexisting benign chondroid lesion. Extraosseous chondrosarcoma arises in soft tissue outside the skeleton. Mesenchymal chondrosarcoma is a high-grade variant that has clinical and microscopic features distinct from those of conventional chondrosarcoma.
CLINICAL AND RADIOGRAPHIC FEATURES
Chondrosarcoma of the general skeleton comprises about 10% of all primary malignant bone tumors, making it about half as common as osteosarcoma. The peak ages of occurrence are between 30 and 50 years.
Chondrosarcoma of the jaws is a rare lesion. Although the peak incidence is at 20 to 40 years, many cases have been reported in old age. The most common chief complaint is a swelling or mass, with pain reported by less than half of patients at the time of initial diagnosis. Maxillary lesions may cause epistaxis or nasal obstruction. The most common locations are the anterior region of the maxilla and the posterior mandible. Dental complaints may cause the patient to seek treatment. These include loosening, resorption, and exfoliation of teeth, as well as movement of teeth and formation of diastemata.
The radiographic appearance of chondrosarcoma of the jaws is variable. It can be radiolucent, radiolucent with radiopaque areas, or diffusely radiopaque. It most commonly presents as a poorly defined radiolucent lesion with radiopaque foci resulting from mineralization or ossification of the cartilage matrix. It occasionally causes symmetric widening of the periodontal ligament space and a “sunburst” pattern. These features are similar to what has been described in osteosarcoma. Occasionally, a chondrosarcoma can infiltrate between trabeculae of normal bone without significantly destroying bone. The intact trabecular bone has an essentially normal radiographic appearance even though the tumor can be extensive.
HISTOPATHOLOGIC FEATURES
The microscopic features of chondrosarcoma vary from almost benign to frankly malignant. Some tumors have abundant cartilage matrix, whereas others have almost none. The neoplastic chondrocytes typically are arranged in nests or groups. The nuclei are variable in size and shape. Many chondrocytes are binucleated: that is, two nuclei are present within one lacuna ( Figure 34-4 ). The number of mitotic figures increases with the increasing grade of the tumor. The cartilage intercellular matrix varies from mature hyaline cartilage that can become calcified to myxoid stroma, which is connective tissue that contains an increased amount of ground substance that results in lightly basophilic staining. Bone may be seen in the tumor, but it is residual or newly formed normal bone made by the host as a reaction to the tumor. There is no bone directly formed by the tumor cells.
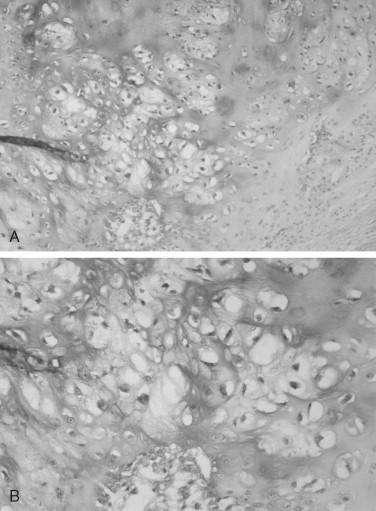
The grading system established for chondrosarcoma is important because it is related to prognosis. A three-grade system is used, with grade 1 the best differentiated. Grade 1 lesions have chondrocytes with nuclei that appear normal or slightly enlarged and occasionally binucleated. Mitotic figures are scarce. Grade 1 lesions cause the most difficulty in diagnosis because of their similarity to chondroma. Grade 2 chondrosarcomas have less matrix and more cells, especially at the periphery of the tumor. The chondrocytes are enlarged, and often there is more than one cell per lacuna. Grade 3 lesions have the greatest cellularity and the least amount of cartilage matrix. The chondrocytes are bizarre in shape, and nuclei are typically greatly enlarged and pleomorphic. Necrosis is present and may be prominent. Mitoses are more numerous in grade 3 tumors but still may be difficult to find. In Garrington and Collett’s review of 37 cases, 46% of the tumors were grade 1, 41% were grade 2, and only 14% were grade 3. Their patients with grade 1 and grade 2 chondrosarcomas had almost the same survival rate, but both groups did much better than patients with grade 3 tumors.
An important concept for oral and maxillofacial surgeons is that chondrosarcomas usually have a dominant microscopic grade but may also contain small foci that are of different grades. If one small incisional biopsy of a tumor is performed, the specimen may demonstrate features that are not representative of the entire lesion. Wide surgical sampling of large tumors is important to obtain the most accurate diagnosis.
Another critical issue is the distinction between chondrosarcoma and chondroma. Some authors do not accept a diagnosis of a benign tumor of cartilage in the jaws and recommend that all cartilaginous tumors of the craniofacial bones, even if microscopically benign, be widely excised. However, cartilage remnants of embryologic development occur in the jaws, most commonly in the anterior maxilla. They are not neoplasms and are benign. It might be safer to state that chondrosarcomas of the jaw are reportedly more common than chondromas. When a surgeon receives a histopathologic diagnosis of chondroma, it is prudent to request a second opinion.
TREATMENT AND PROGNOSIS
Chondrosarcomas of the jaws do not appear to be radiosensitive and have not responded well to chemotherapy. The recommended management is radical surgical excision, bearing in mind that they may extend well beyond the apparent clinical or radiographic margins. Metastasis is uncommon with jaw lesions, and when it does occur, the lung and bone are the most common locations. Radical neck dissection is probably not indicated.
The prognosis for chondrosarcoma of the jaws appears to be influenced by the histologic grade of the tumor and specific site of origin, with maxillary tumors having the worst prognosis and tumors of the anterior mandible having the best survival rate. As with osteosarcoma of the jaws, the prognosis of chondrosarcoma depends upon complete surgical removal of the lesion with clear surgical margins.
The prognosis for chondrosarcoma compared with that of osteosarcoma of the jaws is controversial. A 1995 report from the Mayo Clinic states that chondrosarcoma of the jaws and facial bones is a locally aggressive tumor with a better prognosis than osteosarcoma. Reported survival rate in chondrosarcoma patients was 68% at 5 years and 44% at 15 years. Death was the result of uncontrolled local recurrence rather than metastasis. Other authors report a much poorer prognosis for jaw chondrosarcoma, with a 5-year survival rate of 15% to 20%.
MESENCHYMAL CHONDROSARCOMA
Mesenchymal chondrosarcoma is a rare tumor with sufficiently unique clinical and microscopic features to warrant consideration as a lesion distinct from conventional chondrosarcoma. Up to one third of mesenchymal chondrosarcomas arise in extraskeletal soft tissue. Lesions originating within bone have a predilection for the maxilla, mandible, and ribs. Most patients with this tumor are between the ages of 10 and 30 years. The most common presenting complaints are pain and swelling. Radiographically, the lesion appears as a destructive radiolucent lesion that may contain stippled calcification.
Histopathologic examination of mesenchymal chondrosarcoma reveals two distinct patterns. Sheets of small, undifferentiated round or spindle cells alternate with areas of well-differentiated cartilage. An adequate biopsy sample is necessary to reveal the areas of cartilage, as the small cells may be confused with Ewing’s sarcoma.
The recommended treatment for mesenchymal chondrosarcoma is radical surgical excision. It is a highly malignant tumor, and it metastasizes more frequently than conventional chondrosarcoma. The prognosis is poor, with a 20% to 30% long-term rate of survival.
RADIATION-INDUCED SARCOMA OF BONE
Sarcomas occasionally arise in normal bone previously irradiated for disease in adjacent tissue. Osteosarcoma is the most common type of radiation-induced sarcoma of bone, followed by fibrosarcoma. Many of the cases of postradiation sarcomas reported in the skull and facial bones have occurred in bone with preexisting benign diseases, such as fibrous dysplasia and Paget’s disease. The prognosis for postradiation sarcomas is reportedly worse than that for primary sarcomas.
The average latent period between radiation treatment and development of sarcoma in bone has been reported as varying from 3 months to 53 years, but the mean latency period is 10 to 20 years. The radiation dose has varied from 1600 to 11,200 centigray (cGy). Most sarcomas have occurred after exposure to 5500 cGy, and doses less than 3000 cGy are unlikely to cause radiation-induced sarcoma.
MULTIPLE MYELOMA
Multiple myeloma is a malignant proliferation of plasma cells arising within bone marrow. It is a monoclonal proliferation, meaning the tumor cells are all identical to the malignant cell of origin. The malignant plasma cells produce large quantities of identical nonfunctional immunoglobulins. The immunoglobulin light chain is restricted to the kappa or lambda type.
CLINICAL, RADIOGRAPHIC, AND LABORATORY FEATURES
Multiple myeloma is the most common malignant tumor of bone other than metastatic carcinoma. The mean age at diagnosis is 63 years and occurrence is rare before age 40. It is more common in males.
A common presenting complaint of patients with multiple myeloma is bone pain, especially associated with compression fractures of vertebrae. The bone marrow tumors cause bone resorption, which leads to hypercalcemia in 20% of patients. Destruction of hematopoietic bone marrow can result in anemia and fatigue, thrombocytopenia with purpura and epistaxis, and infection and fever caused by leukopenia. Renal disease results from hypercalcemia and the overwhelming amount of light-chain proteins in the blood.
The most characteristic radiographic appearance in multiple myeloma is multiple, sharply demarcated radiolucent lesions without corticated borders, referred to as “punched-out” lesions ( Figure 34-5 ). Radiolucent lesions with ragged borders can also be seen. Lesions are most common in bones with hematopoietic marrow, such as the skull, vertebrae, clavicles, sternum, ribs, pelvis, and proximal humerus and femur.
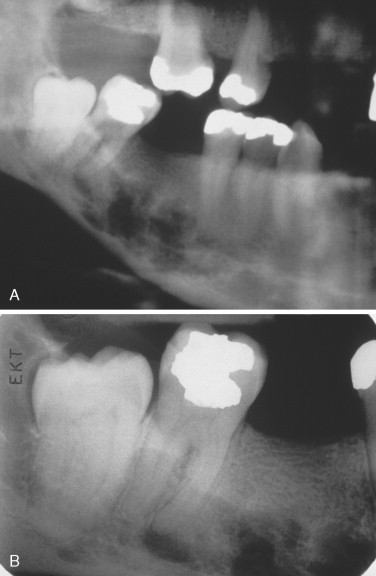
Jaw involvement has been reported in 12% to 30% of cases. Oral lesions may cause pain, swelling, paresthesia, loosening of teeth, hemorrhage, abnormal healing after extractions, mandibular fracture, and soft tissue enlargements.
An important laboratory procedure is serum protein immunoelectrophoresis demonstrating a monoclonal immunoglobulin protein peak, known as a myeloma (M) spike. Sometimes only a monoclonal light chain is produced rather than the entire immunoglobulin molecule. Bence Jones protein is detected in the presence of the monoclonal light chains in the urine. It should be noted that monoclonal proteins are detected more commonly in the serum than is Bence Jones protein in the urine. Other laboratory findings include hypercalcemia and increased erythrocyte sedimentation rate.
About 10% of patients with multiple myeloma have systemic amyloidosis. The amyloid protein in multiple myeloma consists of immunoglobulin light chains. Amyloid may be deposited in the liver, spleen, kidneys, adrenal glands, salivary glands, and skin. Amyloidosis of the tongue has been estimated to occur in 25% of cases. The tongue can be firm and diffusely enlarged or contain multiple discrete nodules of amyloid. Amyloid may also be deposited in the gingiva ( Figure 34-6 ).
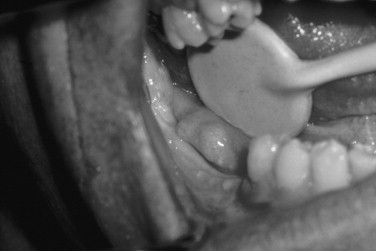
Plasma cell tumors may have two other clinical presentations. One is a solitary lesion of bone called a plasmacytoma . This is rare in the jaws, and an apparent solitary plasma cell lesion of the jaws is almost always part of multiple myeloma. Extramedullary plasmacytoma is a plasma cell tumor of soft tissue. Of these lesions 80% occur in the head and neck, most commonly in the sinonasal region, but they have also been reported in oral mucosa.
HISTOPATHOLOGIC FEATURES
Microscopic examination of a bone marrow aspirate or bone biopsy specimen reveals a monotonous proliferation of plasma cells ( Figure 34-7 ). The neoplastic cells may vary from well-differentiated plasma cells to large, poorly differentiated cells resembling immunoblasts. For jaw lesions it may be difficult, using routine hematoxylin and eosin stains, to determine whether the lesion is a neoplasm of plasma cells or an inflammatory lesion. However, inflammatory lesions have a mixed leukocytic infiltrate, including neutrophils and macrophages. Immunoperoxidase techniques can be helpful in making the distinction between inflammatory and neoplastic lesions. Plasma cell neoplasms demonstrate monoclonal light chains within the cytoplasm of plasma cells, whereas inflammatory lesions show polyclonal light chains.
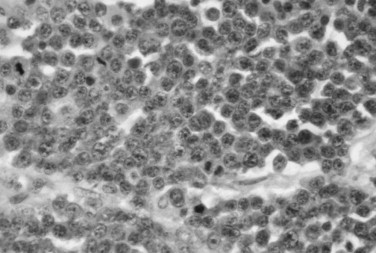
TREATMENT AND PROGNOSIS
The traditional treatment for multiple myeloma uses chemotherapeutic alkylating drugs and steroids. Localized radiotherapy can be given for palliation of painful bone lesions. Bone marrow transplantation has been used for patients who do not respond to chemotherapy.
The median survival time for patients with multiple myeloma is 3 years. The most important predictor of long-term survival is the occurrence of a response to treatment. Causes of death are most commonly infection and renal failure.
EWING’S SARCOMA
Ewing’s sarcoma is a primary malignant tumor of bone that most commonly involves the pelvis and lower extremities. About 4% of all Ewing’s sarcomas occur in the bones of the head and neck. Currently, Ewing’s sarcoma is thought to be of neuroectodermal origin because it demonstrates the same reciprocal translocation of chromosomes 11 and 22 as seen in primitive neuroectodermal tumors.
CLINICAL AND RADIOGRAPHIC FEATURES
The average age of patients with Ewing’s sarcoma of the head and neck is 10.9 years, with almost all cases occurring between the ages of 5 and 30 years. The most frequent presenting features are swelling and pain ( Figure 34-8 ). Loosening of teeth and paresthesia are also common in jaw lesions. Occasionally, fever, leukocytosis, and an elevated erythrocyte sedimentation rate can occur, causing confusion with osteomyelitis.
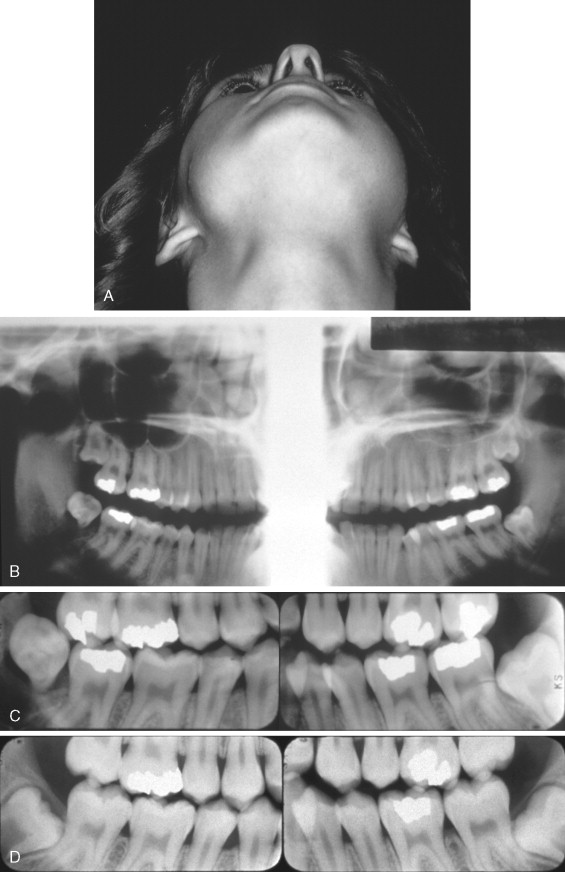
Ewing’s sarcoma appears radiographically as a radiolucent lesion with ragged, poorly defined margins. In the jaws it may perforate the cortex and have an associated overlying soft tissue mass. The classic “onionskin” periosteal reaction described for tumors of the extremities is uncommon with jaw lesions. It should be noted that periosteal reactions can occur in other malignant tumors and also in inflammatory reactions and are not pathognomonic of Ewing’s sarcoma.
HISTOPATHOLOGIC FEATURES
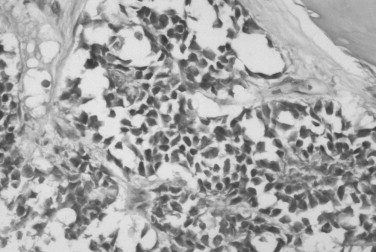
Ewing’s sarcoma consists of small round cells with large, hyperchromatic nuclei; sparse nucleoli and cytoplasm; and poorly defined cell borders ( Figure 34-9 ). Mitotic figures are not abundant. Vascular channels, hemorrhage, and necrosis are relatively common. About 75% of Ewing’s sarcomas contain glycogen granules, but glycogen can also be present in other small round cell tumors. The microscopic differential diagnosis includes metastatic neuroblastoma, small-cell osteosarcoma, embryonal rhabdomyosarcoma, lymphoma, and primitive ectodermal tumor. A panel of immunohistochemical stains is used to exclude other lesions. Ewing’s sarcoma typically stains for CD 99; however, CD 99 can also stain other tumors and is sometimes found in normal tissue.
TREATMENT AND PROGNOSIS
Ewing’s sarcoma is an extremely aggressive neoplasm, which frequently metastasizes to lungs, liver, lymph nodes, and other bones. The prognosis has improved significantly since the introduction of integrated therapies including surgery, multiagent chemotherapy, and radiotherapy. Because of the risk of radiation-induced sarcoma, radiation therapy is typically not used unless the surgical margins are not clear. The location of the primary site of tumor is the single most important predictor of clinical behavior. The prognosis of Ewing’s sarcoma of the head and neck is significantly better than that for other locations.
METASTATIC TUMORS TO THE JAWS
Metastatic tumors are the most common malignancy involving the jaws. The vast majority of metastases are of epithelial origin and thus carcinomas. The mean age of patients is 46 years. The most common locations of the primary tumor are breast, followed by lung, colorectal region, kidney, thyroid, and male and female reproductive systems. The most common location of metastatic tumors in the jaws is the molar area of the mandible. It is important to note that the primary malignant tumor is undiagnosed in almost two thirds of the cases, making the oral metastatic lesion the first evidence of malignancy originating elsewhere in the body.
Patients with metastatic tumors to the jaws may experience bony enlargement, pain, paresthesia, and loosening of teeth. Often the symptoms are poorly defined and mimic dental inflammatory disease. Significantly, some patients may be asymptomatic and have the lesion during radiographic examination.
Radiographic examination most commonly reveals a radiolucent lesion, typically with poorly defined or ragged borders. Occasional lesions demonstrate deceptively wellcircumscribed borders. Carcinomas of the breast, prostate, and thyroid may be radiolucent, radiopaque, or mixed.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


