Clefting of the lip and palate represents a complex malformation of both the hard and soft tissues of the face. Children who are born with a cleft of the lip and/or palate experience several unique functional and esthetic challenges requiring a combined (interdisciplinary) management approach. To achieve the best outcomes, clinicians must take into consideration each child’s speech, occlusion, facial appearance, self-esteem, and a number of other complex issues that arise during the child’s development. Successful reconstruction requires multiple surgical phases. Consequently, treatment that takes place during growth and development must be carried out with concern for the long-term biologic consequences. Thoughtful planning is formulated in order to provide the maximum long-term functional and esthetic benefit to the patient. Surgeons caring for children with clefts must maintain a firm cognitive understanding of the three-dimensional anatomy of the cleft lip and palate malformation and the complex interplay that exists between surgical procedures and ongoing facial growth. Various surgical procedures involved in staged reconstruction of cleft lip and palate have been described extensively in the literature. The American Cleft Palate–Craniofacial Association (ACPA) has developed parameters of care to facilitate the coordinated interdisciplinary treatment of individuals affected with cleft lip and palate deformities. The ACPA document summarizes a management protocol that is centered around thoughtful timing of specific interventions based on the patient’s dental, skeletal, speech, and psychologic development. Contemporary management involves several phases of surgery during infancy (cleft lip repair and palate closure) and early childhood (bone graft reconstruction of the cleft maxilla and alveolus) that are considered required operations in all cases of complete unilateral or bilateral cleft lip and palate. In addition to those primary stages of repair, most children will require additional procedures for treatment of secondary issues. Secondary reconstruction of cleft lip and palate may involve surgery for treatment of velopharyngeal dysfunction, bone graft reconstruction of clefts of the maxilla, correction of residual skeletal disproportion and malocclusion, closure of palatal fistula, normalization of lip and nasal form, and prosthetic rehabilitation of the cleft dental gap. Although indications for each of the primary and secondary surgical undertakings are specific to each child, they must not be viewed as isolated events. Ongoing growth and development in concert with the secondary surgical effects must be considered. This chapter reviews aspects of secondary cleft lip and palate reconstruction that may be required after primary cleft lip and palate repair. The chapter is formulated with the purpose of providing an organized description of the contemporary philosophy based on the most current outcome data, and how this relates to the specific timing controversies of surgical intervention ( Table 42-1 ).
| Surgical Treatment | Age | Timing Considerations |
|---|---|---|
| Cleft lip repair | 10-12 weeks | |
| Cleft palate repair * | 9-18 months | Exact timing of repair is based on child’s speech-language age |
| Secondary palate surgery for velopharyngeal insufficiency | 3-5 years | |
| Bone graft reconstruction of cleft maxilla and alveolus * | 6-9 years | Based on dental development |
| Orthognathic surgery | 14-16 years for girls, 16-18 years for boys | |
| Dental implant placement | 16-18 years | |
| Lip and nasal revision | After 5 years | Varies widely depending on clinical findings and psychosocial concerns; definitive nasal surgery usually delayed until adolescence |
FISTULA CLOSURE
A child born with cleft palate has as part of the congenital deformity a communication between the oral and nasal cavities. Successful surgical repair requires separation of the oral and nasal side soft tissues followed by reconstruction of the soft palate musculature and establishment of separate nasal and oral mucosal linings. The result is two-layer closure of the hard palate (nasal mucosa and oral mucosa) and three-layer closure of the soft palate (nasal mucosa, levator musculature, and oral mucosa). A residual abnormal oronasal communication, or fistula, after initial repair is a relatively frequent issue with many types of palate repair. Before deciding on a specific management approach to the residual fistula, it is important to define the clinical situation based on the patient’s age, previous surgical history, and exact location of the fistula.
The extent to which the cleft defect involves the primary and secondary palate must be determined. The primary palate consists of the anatomic structures anterior to the incisive foramen. The secondary palate consists of the anatomic structures between the incisive foramen and uvula. It follows that a complete cleft of the primary and secondary palate would therefore involve the maxilla, alveolus, hard palate, and soft palate. An isolated cleft palate by definition involves the hard and soft palates (without affecting the alveolus)—i.e., a complete cleft of the secondary palate. A cleft involving only the soft palate (and not the hard palate or alveolus) is described as an incomplete cleft of the secondary palate. Even when a child is born with a complete cleft palate (i.e., affecting the primary and secondary palates), the primary repair involves closure of the secondary palate, or only those structures posterior to the incisive foramen.
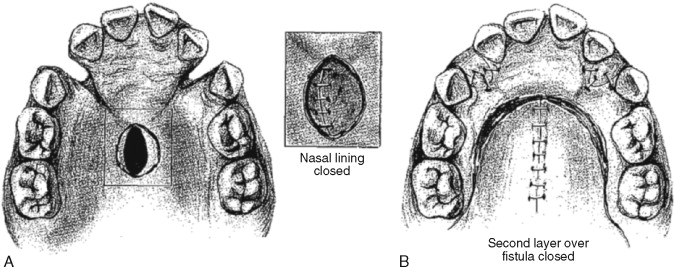
The goals of cleft palate repair during infancy are twofold: first, to establish complete “watertight” closure of the secondary palate for separation of the oral and nasal cavities, and, second, to repair the levator musculature in order to allow for optimal speech production. Repair of the skeletal maxillary alveolar cleft defect and its associated oronasal communication is not generally attempted at this stage. Many surgeons consider this alveolar defect part of the original cleft deformity that has been purposely left and therefore not a true fistula. Definitive repair of the anterior alveolar aspect of the cleft (or nasolabial fistula) is instead incorporated into the bone graft reconstruction performed during midchildhood based on dental development. Ideally, a child with a complete cleft palate will undergo palate repair (successful closure of the hard and soft palates) during infancy, followed by bone graft reconstruction of the maxilla and alveolus (or primary palate) with closure of the residual nasolabial fistula during childhood.
Unfortunately, residual palatal fistulas are occasionally encountered after the initial palate repair. The risk of fistula formation seems to be associated with the size of the original cleft defect and the experience of the surgeon. The type of repair used by the surgeon may also affect the fistula rate. Recent reports indicate that a two-flap palatoplasty technique is associated with the lowest rate (3.4%) of palatal fistula formation. The Furlow double-opposing Z-plasty, is, in most studies, associated with a higher incidence of oronasal fistula.
The most common location of residual palatal fistula occurrence after cleft palate repair is the junction of the hard and soft palates. This is followed by the anterior hard palate and incisive foramen region. The incidence of palatal fistula after single-stage palatoplasty varies greatly, with the reported rates higher than 50%.
INDICATIONS FOR FISTULA REPAIR AND TIMING OF SURGERY
Most fistulas are noted early in the postsurgical period after palate repair. These are the direct result of local wound breakdown secondary to tension, vascular compromise, wound healing problems, or other factors. Another time period when a palatal fistula may be encountered is during phase I (before bone graft) orthodontic treatment, especially if maxillary expansion is undertaken. There is disagreement about the causal relationship of orthodontic expansion and development of palatal fistula. Most experienced cleft surgeons believe that fistula defects discovered during maxillary expansion are small, preexisting oronasal communications and are not caused by orthodontic treatment. Small fistulas present since infancy can be hidden within a narrow palate by collapsed maxillary segments and then become “uncovered” as the maxillary arch form is expanded by orthodontic or orthopedic means. Most are not major functional concerns, and can be addressed at the time of bone grafting.
The recommended timing of fistula closure may vary significantly and remains a controversial topic. Although early intervention is uncommon, some surgeons and cleft teams may advocate aggressive management with early closure of any fistula present after initial palate repair. We prefer to take a more long-range view of these problems and delay surgery for several years when possible (no functional speech and/or feeding-related issues). In infants the closure of a small (up to 4 mm), nonfunctional fistula can generally be deferred until later in childhood. In such cases fistula repair may be incorporated into future procedures such as pharyngeal surgery for velopharyngeal insufficiency (VPI) or bone graft reconstruction of the cleft maxilla and alveolus.
When a larger (>5 mm) fistula is present, there is a greater likelihood that functional concerns will be encountered, such as nasal air escape affecting speech, nasal reflux of food and liquids, and hygiene difficulties. When significant functional issues exist, earlier closure of the persistent fistula is indicated. As part of the decision-making process, surgeons must weigh the benefits of fistula repair against the negative effects of a second palatal surgery (involving stripping of mucoperiosteum) on maxillary growth. Another consideration in timing of fistula closure is the type of repair technique selected for repair. Attempts to close a fistula with local flaps or repeat palatoplasty may be undertaken during infancy and early childhood. On the other hand, in cases in which the use of a regional (e.g., tongue) flap may be preferable, the child must be old enough to cooperate with the perioperative regimen.
INDICATIONS FOR FISTULA REPAIR AND TIMING OF SURGERY
Most fistulas are noted early in the postsurgical period after palate repair. These are the direct result of local wound breakdown secondary to tension, vascular compromise, wound healing problems, or other factors. Another time period when a palatal fistula may be encountered is during phase I (before bone graft) orthodontic treatment, especially if maxillary expansion is undertaken. There is disagreement about the causal relationship of orthodontic expansion and development of palatal fistula. Most experienced cleft surgeons believe that fistula defects discovered during maxillary expansion are small, preexisting oronasal communications and are not caused by orthodontic treatment. Small fistulas present since infancy can be hidden within a narrow palate by collapsed maxillary segments and then become “uncovered” as the maxillary arch form is expanded by orthodontic or orthopedic means. Most are not major functional concerns, and can be addressed at the time of bone grafting.
The recommended timing of fistula closure may vary significantly and remains a controversial topic. Although early intervention is uncommon, some surgeons and cleft teams may advocate aggressive management with early closure of any fistula present after initial palate repair. We prefer to take a more long-range view of these problems and delay surgery for several years when possible (no functional speech and/or feeding-related issues). In infants the closure of a small (up to 4 mm), nonfunctional fistula can generally be deferred until later in childhood. In such cases fistula repair may be incorporated into future procedures such as pharyngeal surgery for velopharyngeal insufficiency (VPI) or bone graft reconstruction of the cleft maxilla and alveolus.
When a larger (>5 mm) fistula is present, there is a greater likelihood that functional concerns will be encountered, such as nasal air escape affecting speech, nasal reflux of food and liquids, and hygiene difficulties. When significant functional issues exist, earlier closure of the persistent fistula is indicated. As part of the decision-making process, surgeons must weigh the benefits of fistula repair against the negative effects of a second palatal surgery (involving stripping of mucoperiosteum) on maxillary growth. Another consideration in timing of fistula closure is the type of repair technique selected for repair. Attempts to close a fistula with local flaps or repeat palatoplasty may be undertaken during infancy and early childhood. On the other hand, in cases in which the use of a regional (e.g., tongue) flap may be preferable, the child must be old enough to cooperate with the perioperative regimen.
OPERATIVE TECHNIQUES FOR CLOSURE OF PALATAL FISTULAS
Repair of residual palatal fistulas using a number of different techniques after cleft palate repair has been described. Current techniques used for fistula repair include local palatal flaps, modifications of the von Langenbeck and two-flap palatoplasty techniques, palatoplasty with incorporation of a pharyngeal flap, and the use of a tongue flap. Other regional flaps, including the buccal mucosal, buccinator myomucosal, temporalis muscle, and vascularized tissue transfers are less frequently used but have been described. Recently the use of acellular dermal matrix as an interpositional barrier has been used as an adjunct to repairing fistulas.
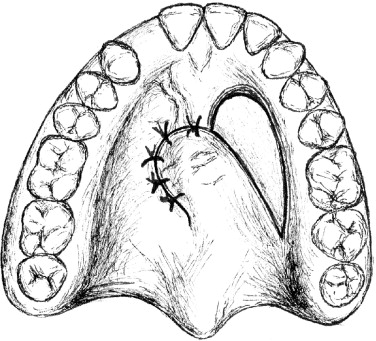
One of the most frequently described procedures for closure of residual fistulas is the use of local soft-tissue flaps created within the palatal mucosa and rotated over the defect for closure ( Figure 42-1 ). The components of this approach are the creation of turnover flaps around the defect for nasal side closure, elevation of a palatal finger flap, and rotation of the flap for coverage of the defect. A significant area of exposed bone is left at the donor site, and this is allowed to heal by secondary intention. Unfortunately, this type of repair is useful only for very small palatal defects and is associated with a relatively high failure rate. Small rotational flaps within palatal tissues that contain extensive scarring from prior surgical procedures are difficult to mobilize without residual tension and may have diminished blood supply, resulting in a less-than-ideal healing capacity and a potentially greater chance of wound breakdown. Our preferred approach to residual palatal fistulas involves the modification of one of the primary palate repair techniques, namely the Bardach (two-flap) or von Langenbeck procedure in conjunction with the use of acellular dermal matrix as an interpositional graft material. These approaches allow adequate coverage of even large defects with the use of bulky soft-tissue flaps, a layered repair of the nasal and oral sides, and a tension-free line of closure ( Figure 42-2 ). In addition, the amount of bone that is left exposed after the repair is minimal to none. This is because the vertical depth of the palatal vault translates into soft-tissue extension medially, and the result is palatal soft-tissue flaps that adequately cover the underlying bone with a layer of dead space between the palatal shelves and the oral mucosa lining. The Bardach (two-flap) palatoplasty is our preferred operation in cases in which the fistula defect is 5 mm or larger. The primary advantage of this approach is the ability to raise large soft-tissue flaps, which can be mobilized easily and allow for visualization and tension-free closure of the nasal mucosa. By comparison, one of the theoretic advantages of the von Langenbeck procedure is the creation of bipedicled flaps that maintain anterior and posterior blood supplies. Although the anterior pedicles do provide additional perfusion, they also result in less freely movable flaps with limited access and visualization of the nasal side tissues. For this reason we use the von Langenbeck technique only for relatively small defects within the hard palate. When there is a much larger (>1.5 cm) defect, successful closure may dictate that the surgeon recruit additional soft tissue using a regional flap.
Fistula defects within the posterior hard palate or soft palate may be addressed with the use of a modified palatoplasty procedure, as described earlier, in combination with a superiorly based pharyngeal flap. After the palatal flaps are developed and the nasal side dissection is complete, a pharyngeal flap is harvested. The pharyngeal flap soft tissue is then incorporated into the nasal side closure of the area where the fistula was present. With this technique a substantial amount of additional soft tissue can be recruited for tension-free repair of a large palatal defect. When the fistula is located within the anterior two thirds of the hard palate and cannot be closed using local tissue with acellular dermal matrix interposed, then the procedure of choice for recruitment of additional soft tissue may be the anteriorly based dorsal tongue flap ( Figure 42-3 ). First the nasal side closure of the palatal defect is performed using turnover flaps with multiple interrupted sutures. Next this technique calls for development of an anteriorly based tongue flap that is approximately 5 cm in length by one to two thirds the width of the tongue. The tongue flap is elevated along the underlying musculature and then inset using multiple mattress sutures for closure of the oral side. The recipient bed within the tongue is closed primarily. After the initial surgery, the tongue flap is allowed to heal and primarily vascularize for approximately 2 weeks. After this time the patient is returned to the operating room. Nasal fiberoptic intubation may be indicated for the second-stage procedure because the tongue is still sutured to the palate, restricting normal visualization of the airway. The flap is sectioned and the stump at the donor site is freshened and inset. The use of laterally or posteriorly based tongue flaps has also been presented in the literature. In our opinion an anteriorly based flap is better tolerated by most patients and allows for the greatest degree of tongue mobility, decreasing risk of tearing the flap from its palatal insertion.
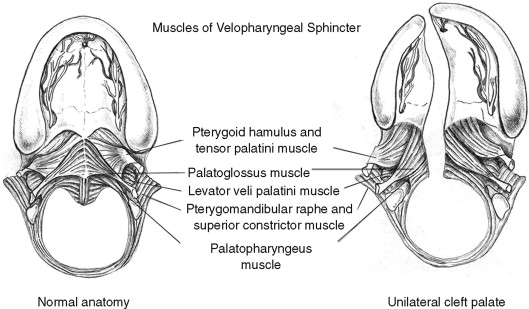
SECONDARY CLEFT PALATE SURGERY FOR MANAGEMENT OF VELOPHARYNGEAL DYSFUNCTION
The secondary palate is composed of a hard (bony) palate anteriorly and a soft palate or “velum” posteriorly. Within the soft palate the levator veli palatini muscle forms a dynamic sling that elevates the velum toward the posterior pharyngeal wall during the production of certain sounds. Other muscle groups within the velum, the tonsillar pillar region, and the pharyngeal walls also affect resonance quality during speech formation ( Table 42-2 ). The combination of the soft palate and pharyngeal wall musculature jointly form what is described as the velopharyngeal mechanism ( Figure 42-4, A ). The velopharyngeal mechanism functions as a complex sphincter valve in order to regulate airflow between the oral and nasal cavities and create a combination of orally based and nasally based sounds. Children born with a cleft palate have, by definition, a malformation that dramatically affects the anatomic components of the velopharyngeal mechanism. Clefting of the secondary palate causes division of the musculature of the velum into separate muscle bellies with abnormal insertions along the posterior edge of the hard palate ( Figure 42-4, B ). The initial palatoplasty is, as described earlier, not simply carried out for closure of the palatal defect (oronasal communication) itself, but is aimed at addressing the underlying muscular anatomic deformity. During two-flap palatoplasty, care must be taken to sharply separate the levator muscle bellies off the palatal shelves, realign them, and establish continuity in order to create a functioning palatal-levator muscle sling. Some describe this primary repair of the palatal musculature as an “intravelar veloplasty.” Although this description helps to articulate the importance of addressing the levator muscle, it may confuse some clinicians by suggesting that muscle repair or “intravelar veloplasty” is a separate procedure. The degree of aggressive retropositioning of the levator musculature can be variable among surgeons. Irrespective of the type of cleft palate repair technique employed (e.g., von Langenbeck, Bardach, Furlow), meticulous release of abnormal levator muscle insertions with velar muscle reconstruction must be incorporated as a critical element of the surgical procedure. Most children who undergo successful cleft palate repair during infancy (9 to 18 months) will go on to develop speech that is normal or demonstrate minor speech abnormalities that are amenable to speech therapy. In a smaller segment of this patient population, the velopharyngeal mechanism will not demonstrate normal function despite surgical closure of the palate. VPI is defined as inadequate closure of the nasopharyngeal airway port during speech production. The exact cause of VPI after “successful” cleft palate repair is a complex problem that remains difficult to define and quantify. Inadequate surgical repair of the musculature is one potential cause of VPI. The role of postsurgical scarring and its impact on muscle function and palatal motion is poorly understood. The theoretic advantages of using a Furlow double-opposing Z-plasty procedure for the initial palate repair include better realignment of the palatal muscles and lengthening of the soft palate. These benefits may be negatively balanced by a velum that demonstrates less mobility secondary to scarring associated with two separate Z-plasty incisions. Even muscles that are appropriately realigned and reconstructed may fail to heal normally and function properly because of congenital defects having to do with their innervation. In addition, a repaired cleft palate is only one factor contributing to velopharyngeal function. Nasal airway dynamics and abnormalities related to vocal tract morphology and lateral and posterior pharyngeal wall motion may contribute to velopharyngeal dysfunction. Certainly, these other structures may also play a positive role in compensating for palatal deformity. For example, a short, scarred soft palate that does not elevate very well may be compensated for by recruitment and hypertrophy of muscular tissue within the posterior pharyngeal wall (“activation of Passavant’s ridge”). The audible nasal air escape that results in hypernasal speech associated with VPI is perhaps the most debilitating consequence of the cleft palate malformation. A variable number of children with VPI after palatoplasty will go on to require management involving additional palatal and pharyngeal surgery. The percentage is quite variable and not universally agreed on but generally is approximately 20-40% for nonsyndromic patients. Patients with syndromes do have higher rates of VPI, but these are generally due to other contributing factors such as cognitive status or neural innervation. Other studies claim much lower rates of VPI, but the measurement and reporting is not uniform or validated in these published studies. Without a truly objective measure of speech in this patient, population, it is difficult to know the true incidence of VPI after repair.
| Muscle | Insertion | Origin | Function |
|---|---|---|---|
| Musculus uvulus | Mucous membrane of soft palate | Palatal aponeurosis | Velar extension |
| Tensor veli palatini | Soft and hard palates | Medial pterygoid plate | Opens auditory tube |
| Salpingopharyngeus | Palatopharyngeal aponeurosis | Torus tubarius | Motion of the lateral walls |
| Superior constrictor | Medial pharyngeal raphe | Velum; medial pterygoid plate | Posterior and lateral wall sphinctering |
| Levator veli palatini | Soft palate | Temporal bone | Elevation of the velum |
| Palatopharyngeus | Soft palate aponeurosis | Pharyngeal wall | Adduction of posterior pillars; sphinctering of velum |
| Palatoglossus | Tongue | Tongue | Retract tongue; antagonistic to the levator during speech |
Left untreated, nasal air escape–related resonance problems will lead to other compensatory speech abnormalities and articulation errors. Warren’s elegant aerodynamic demands theory provides the best explanation of what occurs with severe VPI. His theory states that nasal air escape due to inadequate velopharyngeal closure will cause the patient to articulate pressure consonants at the level of the larynx or pharynx instead of within the oral cavity. These abnormal, compensatory misarticulations further complicate problems with speech formation and decrease speech intelligibility in patients with cleft palate–related VPI.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


