Abstract
Objective
To explore the literature on corona-associated mucormycosis and explain its relevance to dentists and oral surgeons.
Methods
A literature search was carried out to identify reported cases on corona-associated mucormycosis since the start of COVID-19 pandemic.
Results
A review of literature identified 265 published papers on CAM between March 2020 and September 2021. Careful screening of abstracts and full texts revealed 29 studies reporting case series of CAM and, 27 case reports on CAM.
Conclusions
A multitude of factors may be responsible for the alarming rise in the incidence of corona-associated mycosis including reduced access to routine medical services during the pandemic, injudicious use of antibiotics, steroids and nutritional supplements. Risk factors contributing to corona-associated mucormycosis need to be recognized. Dentists and oral surgeons can contribute to early recognition of corona-associated mucormycosis involving the maxilla, and palate. Prioritizing research focus on optimal management of mucormycosis may reduce the morbidity and mortality associated with this debilitating infection.
Highlights
- •
Explores the current literature on corona-associated mucormycosis.
- •
Explains the factors contributing to development of mucormycosis in patients with Corona Virus.
- •
Emphasizes the role of dentists and oral surgeons in recognition and management of rhio-orbital mucormycosis.
1
Introduction
Mucormycosis is a fulminant, potentially fatal, opportunistic fungal infection. It affects immunocompromised patients, and its mortality rate has been reported to be as high as 80%. First described as Phycomycosis by Paltauf in 1885, the term mucormycosis was introduced by Baker in 1957 and still remains in use [ , ]. Currently, Mucorales fungi are the next most common mold pathogens after Aspergillus, leading to invasive fungal disease in susceptible patients.
Mucormycosis is primarily seen in patients with immunosuppression. Due to the increasing incidence of diabetes mellitus, cancer, and growing number of organ transplantations, the number of patients at risk for this deadly infection is increasing. Despite aggressive therapy including surgical debridement and adjunct antifungal therapy, morbidity and mortality following mucormycosis remains high. New strategies to prevent and treat mucormycosis are warranted. A better understanding of the pathogenesis and host response to mucormycosis may provide targets for novel therapeutic interventions in the future [ ].
Based on clinical presentation and involvement of typical anatomic regions, mucormycosis is classified into five clinical types namely, (i) rhino-cerebral, (ii) pulmonary, (iii) cutaneous, (iv) gastrointestinal, and (v) disseminated. These categories of invasive mucormycosis tend to occur in patients with specific defects in host defense. For example, patients with diabetes mellitus (DM) complicated by ketoacidosis typically develop the rhinocerebral form of the disease, and much more rarely develop pulmonary or disseminated disease [ ].
The rhino-orbito mucormycosis (ROM) is the most common type of mucormycosis [ , ]. The disease process starts with inhalation of the fungus into the paranasal sinuses. The fungus may spread to invade the palate, maxilla, orbit, or centrally to involve the brain. If untreated, infection usually spreads from the ethmoidal air sinuses to the orbits, resulting in the loss of extraocular muscle function and proptosis [ ]. Classically, the clinical presentation has been described as an orbital cellulitis with proptosis, and neuropathies including loss of vision.
ROM frequently involves the palate and maxilla ( Fig. 1 ). Necrotic exposure of the palatal bone may the first clinically visible manifestation of the disease and patients may initially present to dentists or oral and maxillofacial surgeons. Similarly, neuropathies involving the trigeminal or facial nerve may initially present to oral and maxillofacial surgeons ( Fig. 2 ).
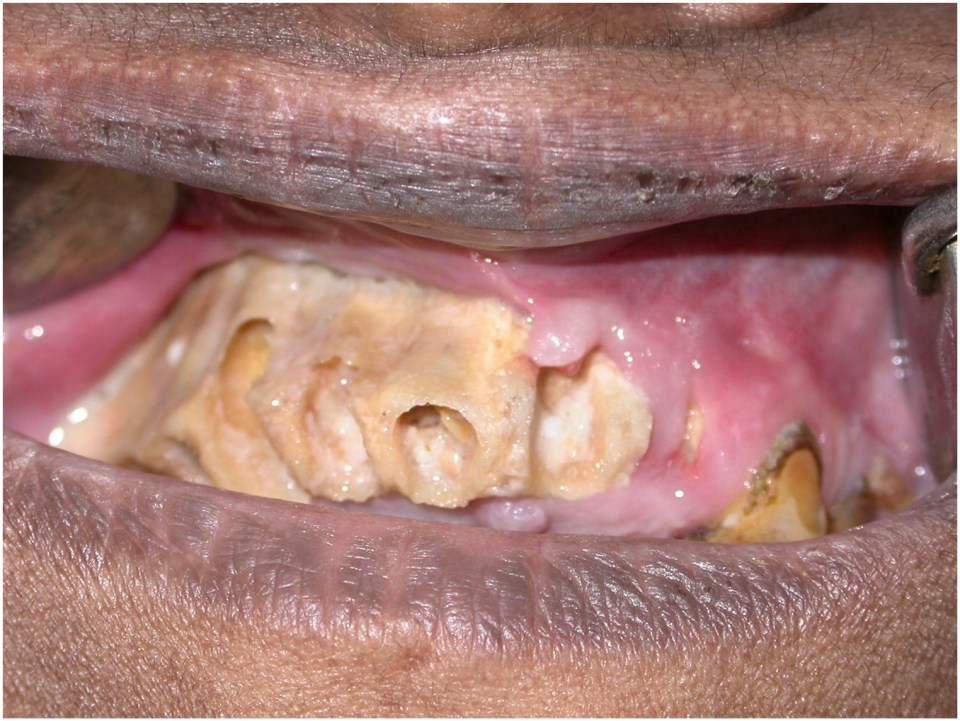

Optimal management of mucormycosis is complicated due to lack of well-defined predictors of clinical response to interventions and high risk of morbidity. Mucormycosis is more commonly reported from countries with poor socio-economic profile and has not received adequate focus in contemporary clinical research. Diagnosis of mucormycosis is based on clinical assessment, and relevant laboratory investigations [ ]. Imaging techniques can provide useful information regarding the extent of specific organ involvement but are not conclusive. Histopathology, microbial identification with polymerase-chain reaction (PCR) tests are the key investigations to establish a definitive diagnosis [ ].
Current management options focus on control of risk factors and a combination of anti-fungal therapy and surgical debridement of infected tissues. Owing to its broad-spectrum antifungal activity, traditionally amphotericin B has been the antifungal agent of choice for the treatment of mucormycosis [ ]. Other options include posaconazole and isavuconazole [ ]. Adjunctive measures to manage mucormycosis include irrigation of infected tissues with Amphotericin B, hyperbaric oxygen therapy and iron chelators. However, lack of robust evidence precludes their routine use in the management of mucormycosis.
The aim of this paper is to review current evidence on Corona-associated mucormycosis (CAM) following the start of COVID-19 pandemic.
2
Review of literature on corona-associated mucormycosis
This paper is based on a review of published literature and did not involve collection of new data. Therefore, the study was exempt from ethics approval. Images used in this paper were obtained after an informed consent from patients.
A review of literature identified 265 published papers on CAM between March 2020 and September 2021. Careful screening of abstracts and full texts revealed 29 studies reporting case series of CAM and, 27 case reports on CAM. Table 1 summarizes the key features of CAM reported in case series. M of studies from reporting CAM were from India.
| Author; Year & Location | Number of cases (N) | Mean Age (Years) | Gender | Clinical Presentation |
|---|---|---|---|---|
| Fouad et al. ., 2020, Egypt | 6 | 51.2 | M = 6 | ROCM |
| Arora et al., 2021, India | 60 | 57 | M = 45; F = 15 | ROCM |
| Ashour et al., 2021, Egypt | 8 | 53.63 | M = 5, F = 3 | AIFS |
| Bayram et al., 2020, Turkey | 11 | 73.1 | M = 9; F = 2 | ROM |
| Bhanuprasad et al., 2021, India | 164 | 50.52 | M = 101; F = 36 | ROCM |
| Desil et al., 2021, India | 100 | 56 | M = 64; F = 36 | ROM |
| Deve et al., 2021, India | 58 | 55 | M = 44; F 14 | ROCM |
| Dubey et al., 2021, India | 55 | 53; | M = 35, F = 20 | ROCM |
| Elhamamsy et al., 2021 Multiple countries | 3 | 67 | M = 3 | ROCM |
| Gupta et al. ., 2020, India | 56 | 53 | M = 41, F = 15 | ROCM |
| Mishra et al., 2021, India | 32 | 58.28 | M = 17; F 15 | ROM = 19; SM = 13 |
| Mitra et al. ., 2021,India | 32 | 57 | M = 23; F = 9 | ROCM |
| Moorthy et al. ., 2020, India | 16 | 54.6 | M = 15, F = 1 | ROCM |
| Nair et al., 2021, India | 127 | 35.9 | Not reported | ROM |
| Nehara et al., 2021, India | 5 | 59 | M = 1; F = 4 | ROM |
| Pakdel et al., 2020, Iran | 15 | 52 | M = 10; F = 5 | ROM |
| Pradhan et al. ., 2021, India | 46 | 48.80 | M = 40, F = 6 | ROM = 33; RCM = 10; Mandibular = 1; SM = 2 |
| Ramaswami et al., 2021, India | 70 | 44.5 | M = 42; F = 28 | ROM |
| Ravani et al., 2021, India | 31 | 56.3 | M = 20, F = 11 | ROM |
| Roa et al., 2021, India | 28 | 49.1 | M = 22; F = 8 | PNS and maxillary involvement |
| Saidha et al., 2021, India | 6 | 39 | M = 4; F = 2 | PNS involvement |
| Sarkar et al., 2021,India | 10 | 67 | M = 8; F = 2 | OM |
| Selarka et al. ., 2021, India | 47 | 55 | M = 35; F = 15 | Maxillary and PNS involvement |
| Sen et al., 2020, India | 6 | 60.5 | M = 6 | ROM |
| Sen et al., 2021, India | 2826 | 51.9 | M = 1993; F = 833 | ROCM |
| Sharma et al., 2020, India | 23 | Not reported | M = 15; F = 8 | SM |
| Singh et al., 2021, India | 13 | 38 | M = 10; F = 3 | ROM = 8; RC = 2; PNS = 2; Pulmonary = 1 |
| Veisi et al. ., 2021, Iran | 2 | 40 | M = 1; F = 1 | ROM |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


