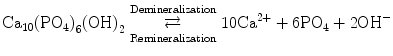Beta Tricalcium Phosphate (β-TCP, Ca3(PO4)2)
β-TCP serves as a bioactive source of mineralizing components and has been used as a Class II device used in facilitating bone remodeling in maxillofacial procedures (FDA, 2005) and orthopedic applications (FDA, 2003). β-TCP can be functionalized with organic and/or inorganic to form funcionalized β-TCP (
fβ-TCP). It has been reported that the combination of fluoride and
fTCP produces stronger, more acid-resistant minerals relative to fluoride, native β-TCP, or
fTCP alone [
60]. As a low-dose system,
fTCP does not rely on high levels of calcium and phosphate to drive remineralization [
60].
fβ-TCP provides a barrier that prevents premature fluoride–calcium interactions and aids in mineralization when applied
via common preparations and procedures [
60]. The combination of fluoride plus
fTCP has been used to remineralize enamel lesions. Karlinsey et al reported that the combination of NaF (
i.e., 500, 950, 1,100, or 5,000 ppm F
−) plus
fTCP in a simple aqueous solution can significantly remineralize white spot enamel lesions relative to that achievable with fluoride alone [
60]. In addition, when added to commercial mouth rinse and dentifrice containing fluoride,
fTCP provided significantly greater fluoride uptake and rehardening relative to a fluoride-free and controlled fluoride-only mouth rinse and dentifrice [
61]. These studies demonstrate that since
fTCP can enhance fluoride-based nucleation activity with subsequent remineralization driven by dietary and salivary calcium and phosphate, the combination of fluoride and
fTCP appears to be a promising approach to remineralization of dental hard tissues.
Currently, nanoscale β-TCP has been used for bone tissue regeneration due to its higher compressive strength, degradation rate, osteoconductivity, and protein absorption compared to submicron β-TCP [
62]. Thus, combination of fluoride and nanomaterials of β-TCP may achieve more effective remineralizing results. However, since β-TCP is often added to mouth rinse and dentifrice, the toxicity of nanoscale β-TCP should be evaluated adequately.
Hydroxyapatite (HAP) Nanoparticles
Synthetic HAP is a biocompatible material, and nano-sized HAP (n-HAP) is similar to the apatite crystal of tooth enamel in morphology and crystal structure. Therefore, it is logical to consider n-HAP as compound substitute for the natural mineral constituent of enamel, with which defects of dental enamel would be repaired.
It has been reported that n-HAP particles can remineralize initial submicrometer enamel caries [
63,
64]. If the dimensions of the n-HAP particles are adapted to the scale of the submicrometer- and nano-sized defects, the reparation of the enamel surface can be greatly improved by using these n-HAP particles. It is shown that the basic building blocks of enamel are 20–40 nm HAP nanoparticles [
65].
In vitro data indicate that n-HAP with a size of 20 nm fits well with the dimensions of the nanodefects on the enamel surface caused by acidic erosion [
64]. Under
in vitro conditions, these n-HAP particles can strongly attach to the demineralized enamel surface and inhibit further acidic attack [
64]. Thus, the use of well-sized n-HAP particles similar to the scale of the natural building blocks of enamel could
de novo repair early carious lesions and thus can protect them from further demineralization to form visible cavities. In the other study, an enamel-like nanocrystal layer with 10 μm thickness in small cavities was achieved
in vitro by pasting fluoride-substituted HAP on the enamel within 15 min, but this process was carried out under pH 3.5 and high concentrations of hydrogen peroxide [
66]. In view of the real conditions of the oral cavity and potential toxicity of n-HAP, the effect of direct use of n-HAP particles on remineralization of enamel should be further investigated and confirmed in a clinical trial.
n-HAP powder can be also added to dental restorative materials for remineralization effects and improvement of mechanical properties due to its excellent biocompatibility and bioactivity [
67,
68]. For instance, compared with micro-HA added to glass ionomer cement, 10 % n-HAP particles (60–100 nm) are incorporated in resin-modified glass ionomer cement, which results in an increased resistance to demineralization and acceptable bonding strength with the only drawback of exceeding the clinically suitable maximum setting time [
69–
71]. Furthermore, the addition of n-HAP and nanofluorohydroxyapatite (n-FHA) to glass ionomer cements increases the compressive, diametral tensile, and biaxial flexural strength of glass ionomer cements [
72,
73]. Besides, the glass ionomer cement containing n-FHA has the potential to increase the amount of fluoride release [
74].
Nanoparticles of HAP have been incorporated into toothpastes or mouth-rinsing solutions to facilitate the remineralization of demineralized enamel or dentin by depositing HAP nanoparticles in the lesions. Commercially available dental prophylactic products containing biomimetic carbonate hydroxyl apatite nanoparticles have been used to fill microdefects on demineralized enamel or dentin surfaces and proved to be effective
in vitro after a 10 min application. However, these promising effects need a clinical study to support them. In addition, the toothpastes with either spheroidal or needle-like particles of n-HAP show better remineralization effect on demineralized enamel than sodium fluoride solutions [
75]. However, the
in vitro study simulating the real conditions of oral cavity or an
in vivo study is needed to further test to prove the remineralization effects of these toothpastes.
Recently, some studies indicated that biomimetic synthesis of hierarchically organized enamel-like structures composed of n-HAP would be an ideal approach to repair enamel microcavities. In the presence of organic additives [
76–
85] or by using various hydrothermal conditions, the
in vitro formation of enamel-like microstructures can be achieved. Formation of enamel-like structures in presence of amelogenin, a major extracellular matrix protein in physiological enamel development, has been well documented. Amelogenin oligomers mediate the self-assembly of oriented parallel needle-like apatite bundles to form nano- and microstructured materials, which is compositionally and morphologically similar to natural enamel [
25,
76,
78–
81,
83,
84,
86–
88]. Amelogenin remineralizes etched enamel surfaces by forming a mineral layer containing needle-like fluoridated HAP crystals with dimensions of 35 nm [
80]. Additionally, self-assembling anionic β-sheet peptides, mainly composed of glutamic acid and glutamine, form fibrillar networks as scaffolds to be mineralized and could enhance remineralization and inhibit demineralization of the enamel [
82]. Surfactants also can work as micelles or microemulsions to mimic the biomineralization process during the formation of enamel [
84]. HAP nanorods modified with monolayers of surfactants can self-assemble into a prism-like enamel structure due to specific surface characteristics [
84].
Although some promising
in vitro results were obtained, the stability and the mechanical properties of the n-HAP and the enamel-like materials are not sufficient for tooth restorations, and the long time (from several hours to days) for the formation of the mineral structures also limits their clinical application [
76,
81]. Therefore, besides remineralization functions, further research should improve the properties of the materials related to clinical operations, thus providing clinically conceivable biomimetic tooth repair.
Amorphous Calcium Phosphate (ACP) Nanoparticles
Amorphous calcium phosphate (ACP) is the initial solid phase precipitating from a highly supersaturated solution with respect to calcium phosphate, which is firstly described by Aaron S. Posner in the mid-1960s [
89]. The morphology of ACP particles is shown as small spheroidal particles in the nanoscale (40–100 nm). Owing to its excellent bioactivity, high cell adhesion, adjustable biodegradation rate, and good osteoconduction, ACP has been widely applied in biomedical fields, especially in orthopedic and dental fields [
90–
93]. Since ACP can convert readily to stable crystalline phases such as octacalcium phosphate (OCP) or HAP, it is difficult to directly use ACP to remineralize dental hard tissues unless stabilized in some way. Therefore, like the nanomateirals of CaF
2 and HAP mentioned above, ACP nanoparticles, as source of calcium and phosphate ions, have also been added to composite resins, ionomer cements, and adhesives. Taking advantage of the ability of ACP to release calcium and phosphate ions, these composites, especially in the acidic oral environment, present remineralization effects on dental hard tissues to prevent secondary caries after restorations. A study using in situ caries models of humans indicated that nanoACP-containing nanocomposites prevented demineralization at the restoration–enamel margins, producing lower enamel mineral loss compared with the control composite [
94]. This result could be attributed to the oral biofilm exposed to nanoACP with higher calcium and phosphorus concentrations than that exposed to the control composite [
94]. This high local concentration at the surface thus stimulates precipitation and deposition into tooth structures as apatite mineral. The remineralizing potential of ACP composites can be improved by introducing Si or Zr elements during low-temperature synthesis of the filler [
95]. Si and Zr ACPs increased the duration of mineral ion release by slowing down the intracomposite ACP to HAP conversion [
96].
Although ACP-containing composites show remineralization ability, these composites exhibit inferior mechanical properties, durability, and water sorption characteristics due to the addition of ACP [
97]. These problems could be attributed to the uncontrolled aggregation of ACP nanoparticles along with poor interfacial interaction between them [
98]. Currently, stabilizing and coupling agents are used to stabilize and disperse ACP nanoparticles in the composites. It was found that anionic surfactants can stabilize the amorphous solid phase against the conversion to apatite during the precipitation of ACP; the particle size of ACP was also moderately reduced. The hydrophilic polyethylene oxide (PEO) is water compatible due to its multiple hydrogen bonding interactions with water molecules and stabilizes ACP nanoparticles by multiple chelation. Thus, the incorporated PEO in ACP fillers can prevent ACP nanoparticles from aggregating and affect the water content of the ACP-containing composites, which eventually will impact both ion release kinetics and mechanical stability of composites [
99].
It has been suggested that ACP works as a precursor to bioapatite and as a transient phase in biomineralization [
100]. This process is thought to be mediated by noncollagenous proteins, such as amelogenin, dentin matrix protein (DMP1), and dentin phosphophoryn (DPP, DMP2) with highly phosphorylated serine and threonine. They are biological stabilizers by chelating calcium ions to control the transformation of ACP to HAP. Therefore, it is possible to develop a biomimetic remineralizing strategy for reparation of teeth caries by mimicking the biomineralization process. In the next section, the development of nanocomplexes of stabilizers and ACP will be reviewed.