Fig. 17.1
Photomicrographs of dental pulp-like tissues formed when SHED cells mixed with scaffolds (PuramatrixTM, 3-D Matrix Technology, Waltham, MA (a) or rhCollagen type I (b)) were injected into the root canals of human premolars and transplanted into immunodeficient mice. A vascularized connective tissue with histological features similar to those observed in normal human dental pulps occupied the pulp chamber. Higher cell density was observed along the dentin walls. Freshly extracted human premolars were used as positive controls (c) (Courtesy of Dr. Vinicius Rosa, National University of Singapore)
Knowing that VEGF is a key proangiogenic factor [92, 93], and that VEGF induces the differentiation of dental pulp stem cells into endothelial cells [15, 17], much interest has been placed in the functionalization of scaffolds with VEGF [90, 94]. Alternatively, it has been shown that a scaffold of collagen type I gel containing dental pulp cells and angiogenic growth factors (i.e., fibroblast growth factor-2, VEGF, and PDGF conjugated to gelatin microspheres) injected into pulpless tooth chambers mediated revascularization and allowed for dental pulp tissue regeneration in vivo [95]. Ongoing studies are attempting to define the optimal concentration of VEGF that promotes the differentiation of dental pulp stem cells into vascular endothelial cells and the recruitment of blood vessels from the periapical region, while at the same time does not elicit excessive vascular edema and increased interstitial pressure with deleterious consequences to the overall viability of the pulp tissue.
A recent study has challenged the need for scaffolds in dental pulp tissue engineering applications. The Sfeir group has recently developed self-assembled, scaffoldless, three-dimensional tissues that were engineered from dental pulp cells [96]. Tissue sheets formed by dental pulp cells are induced to “roll” in culture to form cylinders that are then transplanted into the root canals. In this case, the cells themselves secrete their extracellular matrix forming their own three-dimensional microenvironment and precluding the need for scaffold materials.
In general, cell-based approaches have the advantage of enabling the engineering of tissues with morphology and function that closely resemble those observed in normal human dental pulps. However, these approaches have the intrinsic challenge of requiring the transplantation of cells, which is always associated with increased risks of transmission of pathogens or ex vivo transformation of cells leading to uncontrolled tissue growth [97]. Both are serious, potentially life-threatening issues that must be taken into account before such procedures are routinely used in humans. In an attempt to minimize such risks, several cell-free approaches have been proposed, as follows.
17.4.2 Cell-Free Approaches
Cell-free approaches rely on the recruitment of cells from the periapical region into the pulp chamber as a means to regenerate the pulp of necrotic teeth. Pioneering work by Trope and colleagues designed a protocol in which they create a blood clot by intentional instrumentation of the root canal beyond the apex [98, 99]. This procedure, also called revascularization or regenerative endodontics, has the advantages of not requiring cell harvesting, ex vivo expansion, and transplantation (Fig. 17.2a–c).
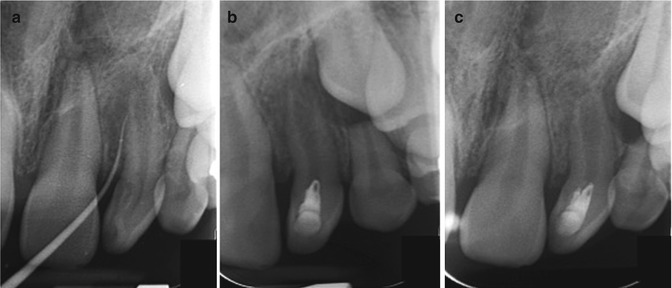

Fig. 17.2
Clinical case demonstrating positive outcome in cell-free regenerative endodontic therapy. (a) Illustrates pretreatment phase of upper left lateral incisor with dens invaginatus. (b) Illustrates at-treatment (immediate postoperative) phase with stimulation of blood clot at apical foramen and coronal seal with MTA and resin restoration. And (c) illustrates apical closure at 6 months posttreatment phase (All courtesy of Dr. Matthew G. Healy and Dr. Tatiana Botero, Endodontic Department, University of Michigan, School of Dentistry)
In general, the regenerative endodontics approach involves the opening of the pulp chamber, followed by instrumentation with copious irrigation. After disinfection, a blood clot is generated by instrumentation beyond the apex with the intent of forming a “scaffold” for the ingrowth of new tissue in the pulp chamber via cell homing induced by molecules naturally released from the blood clot. This is then followed by coronally sealing the canal with mineral trioxide aggregate (MTA), which acts as a hard barrier from pathogenic agents in the oral cavity after the canal has been disinfected. Case reports and clinical studies in humans are currently exploring the safety and efficacy of the revascularization procedure in young permanent teeth [98, 100–102].
Recently, the Mao laboratory suggested a method to exploit chemotaxis as a strategy to guide cells from the periapical region into the pulp chamber as a strategy for regeneration of dental pulps [103]. They showed that injection of a collagen gel solution containing basic fibroblast growth factor (bFGF), VEGF, and platelet-derived growth factor (PDGF) together with nerve growth factor (NGF) and bone morphogenetic protein-7 (BMP7) as a chemotactic was capable of attracting cells into the pulp chamber and regenerating a dental pulp tissue. Using this type of approach, it is possible that dental pulp regeneration is achieved with an injectable scaffold containing a predetermined “cocktail” of chemotactic factors that recruits host cells capable of organizing themselves into a newly formed pulp-like tissue into the root canal.
In general, strategies for dental pulp tissue regeneration that do not require cell transplantation have less regulatory constraints and can be more quickly translated into clinical practice. However, so far the attempts to use cell-free based approaches have not consistently resulted in the formation of a functional dental pulp tissue throughout the full extension of the root canal, when teeth are examined histologically in humans. Under these circumstances, the long-term outcome of teeth treated with cell-free approaches for dental pulp tissue regeneration of necrotic teeth is still rather unclear.
17.5 Challenges Ahead
The seminal discovery that dental pulps contain a subpopulation of proliferative stem cells endowed with multipotency and self-renewal capacities opens the possibility that such cells can be harnessed to regenerate a living pulp in necrotic teeth. However, before this “dream” can be fulfilled, there are several challenges that will have to be overcome (Fig. 17.3), which include:
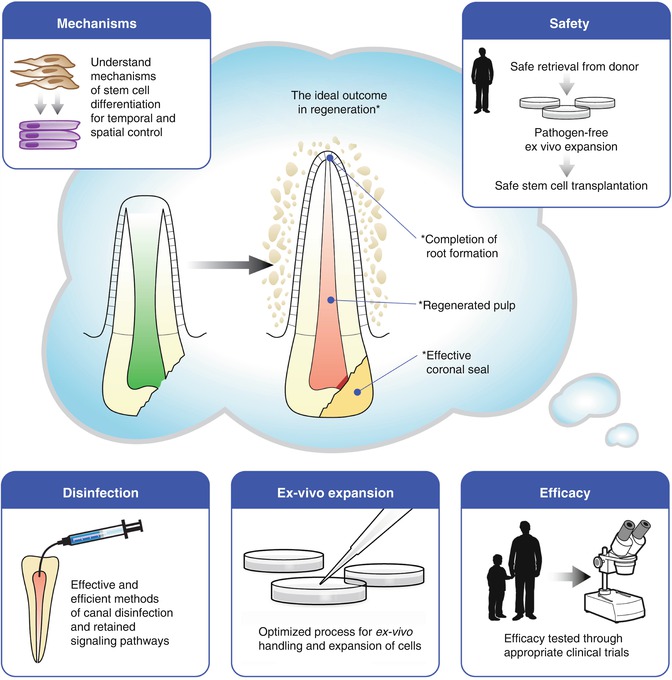

Fig. 17.3
Diagram depicting challenges to the clinical translation of regenerative endodontics procedures: ensure safety of cell-based approaches for dental pulp tissue regeneration; confirm efficacy and long-term outcomes of these procedures through randomized prospective clinical trials; understand mechanisms of stem cell differentiation to enable optimization of dental pulp tissue regeneration in the clinic; effective methods for canal disinfection without compromising dentin-derived morphogenic signals will have to be developed; development of appropriate processes (i.e., ex vivo expansion) that are clinically relevant in the context of contemporary dental practices
A.
Stem cell transplantation has to be done in a highly controlled process that ensures safety in each step. This multistep process includes the retrieval of stem cells from the donor tooth, ex vivo expansion of these cells using pathogen-free processes and reagents, and transplantation to the recipient patient. At each one of these steps, there are potential risks involved, such as contamination of the cells with pathogenic bacteria/fungi and transformation of the cells during ex vivo expansion. Nevertheless, stem cell transplantation has been increasingly used in medicine as standard of care for select procedures, and more recently it began to be used in the context of oral and craniofacial disease [104, 105].
B.
The efficacy of dental pulp stem cell transplantation in the regeneration of new functional dental pulps in humans will have to be verified in randomized, controlled feasibility trials. Preclinical studies have suggested that it is possible to regenerate a dental pulp throughout the length of human roots transplanted in immunodeficient mice [74]. However, before such procedure can become clinically acceptable, well-designed clinical trials will have to be performed showing that DPSC transplantation consistently and effectively generates new dental pulps that remain viable and functional for extended periods of time.
C.
Better mechanistic understanding of the processes regulating dental pulp stem cell differentiation upon transplantation is necessary to optimize the procedure. For example, it is widely accepted that rapid vascularization is a critical step for successful tissue engineering. However, the anatomical constraints of root canals pose significant challenges to the establishment of a functional capillary network in an engineered dental pulp, since vascularization is exclusively achieved through the apical foramina system. It is known that dental pulp stem cells can differentiate into functional blood vessels, in addition to their known differentiation potential into odontoblasts [15, 17]. While this multipotency can be exploited to use dental pulp stem cells as a single cellular source for pulp tissue engineering, one will have to find optimal ways to control the timing of this differentiation process in such a way that enables vascularization while not exhausting the source of odontoblastic precursor cells. Such temporal and spatial control of the differentiation process will require exquisite understanding of the mechanisms involved.
D.
Considering the fact that pulp tissue engineering would typically be considered in the treatment of necrotic/infected teeth, one will have to develop ways to clean up the root canal and eliminate bacterial infection without disrupting the morphogenic signaling pathways that are initiated by dentin-derived proteins (e.g., BMP2) and that are required for odontoblastic differentiation of dental pulp stem cells. For example, it has been shown that excessive use of sodium hypochlorite degrades dentin proteins and prevents dentin-induced odontoblastic differentiation of SHED cells [14]. Recent studies have explored the effects of alternate disinfectant solutions for irrigation of root canals on the survival and differentiation of stem cells [106–108]. Importantly, once a disinfected environment has been prepared within the root canal to receive stem cells, one will also need to maintain it sterile through the use of a coronal sealing strategy that impedes bacterial contamination while maintaining stem cell viability. Materials such as ProRoot mineral trioxide aggregate (MTA, Dentsply Tulsa Dental Specialties) and Biodentine (Septodont) have been considered good candidates for the coronal sealing of teeth that received stem cell transplantation. However, long-term clinical studies are yet to be performed to determine the outcomes of disinfectant solutions and sealing materials. Unquestionably, such studies constitute critical steps toward the clinical use of stem cell-based therapies in endodontics.
E.
And finally, the process for stem cell-based pulp regeneration will have to be clinically relevant in the context of contemporary dental practices. This means that it will have to be done with equipment and materials that are adequate to the context of dental clinic settings. One envisions the possibility of the dental clinician sending the donor tooth to an external laboratory that is capable of expanding the stem cells under Good Manufacturing Practice (GMP) standards, defined by the Food and Drug Administration (FDA) as ex vivo manipulation of clinical-grade cells that are safe and effective for human use. After the ex vivo expansion, cells would be resuspended in an injectable matrix and sent back to the clinician for transplantation into the root canal. Notably, this procedure would have to be done in a cost-efficient way to become an attractive treatment option to the patient.
17.6 Conclusions
While recognizing the many challenges facing the clinical translation of approaches aiming at the regeneration of functional pulp tissues, the authors express guarded enthusiasm that within a reasonable time frame this procedure may become a clinical reality. The need for safe and effective regenerative endodontics procedures comes from the realization that today’s dentistry does not have ideal and long-lasting solutions for some clinical problems, such as necrosis of young permanent teeth with incomplete root formation. While the standard of care in 2014 (i.e., apexification with calcium hydroxide or MTA) allows for disinfection of these roots, it does not enable completion of root formation leaving behind structurally weak teeth with relatively poor long-term prognosis. The engineering of a functional and living dental pulp throughout the full extent of the root canal can potentially enable completion of root formation and improve the long-term outcomes of these immature necrotic teeth.
References
1.
Bressan E, Ferroni L, Gardin C, Pinton P, Stellini E, Botticelli D, Sivolella S, Zavan B. Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS One. 2012;7(11):e49146.PubMedCentralPubMed
2.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–30.PubMedCentralPubMed
3.
Thesleff I, Aberg T. Molecular regulation of tooth development. Bone. 1999;25(1):123–5.PubMed
4.
Peters H, Balling R. Teeth. Where and how to make them. Trends Genet. 1999;15(2):59–65.PubMed
5.
Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238(1):120–32.PubMed
6.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Gehron Robey P, Shi S. Stem cell properties of human dental pulp Stem cells. J Dent Res. 2002;81(8):531–5.PubMed
7.
Fitzgerald M, Chiego Jr DJ, Heys DR. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol. 1990;35:707–15.PubMed
8.
Yamamura T. Differentiation of pulpal cells and inductive influences of various matrices with reference to pulpal wound healing. J Dent Res. 1985;64:530–40.PubMed
9.
Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184(3–4):105–16.PubMed
10.
Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E. Mesenchymal stem cells in the dental tissue: perspectives for tissue regeneration. Braz Dent J. 2011;22(2):91–8.PubMed
11.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12.PubMedCentralPubMed
12.
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–55.PubMedCentralPubMed
13.
Casagrande L, Cordeiro MM, Nör SA, Nör JE. Dental pulp stem cells in regenerative dentistry. Odontology. 2011;99(1):1–7.PubMed
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


