Introduction
The nasal septum is thought to be a primary growth cartilage for the midface and, as such, has been implicated in syndromes involving midfacial hypoplasia. However, this internal structure is difficult to study directly. The aims of this study were to provide direct, continuous measurements of the growth of the nasal septal cartilage and to compare these with similar measurements of the nasofrontal suture to test whether the growth of the cartilage precedes the growth of the suture and whether the growth of the septal cartilage is constant or episodic.
Methods
Ten Hanford minipigs were used. Linear displacement transducers were implanted surgically in the septal cartilage and across the nasofrontal suture. Length measurements of the cartilage and suture were recorded telemetrically each minute for several days.
Results
The growth rate of the nasal septal cartilage (0.07% ± 0.03% length/h) was significantly higher than that of the suture (0.03% ± 0.02% length/h) ( P = 0.004). The growth of both structures was episodic with alternating periods of growth (5-6 per day) and periods of stasis or shrinkage. No diurnal variation in growth of the cartilage was detected.
Conclusions
These results are consistent with the notion that growth of the septal cartilage might drive growth of the nasofrontal suture. Growth of the midface is episodic rather than constant.
The role of the nasal septal cartilage in powering midfacial growth was popularized by Scott, who described the cartilaginous septum as a primary cartilage representing the anterior extension of the cranial base. He suggested that like other primary cartilages the septum would act as a growth center, and its expansion would separate the facial sutures, causing them to grow. Although it is not relevant for transverse midfacial growth in the sagittal plane, Scott’s suggestion has been supported by various findings: (1) the septal cartilage’s ability to grow in organ culture and as an autograft, (2) its response to growth factors and hormones, and (3) the development of midfacial retrognathia in rats injected with septal cartilage antisera. In addition, midfacial retrognathia associated with some anomalies such as Binder’s syndrome, achondroplasia, and arhinencephaly has been attributed, in part, to defects in the nasal septal cartilage. Support for the role of the septal cartilage in powering midfacial growth also comes from extirpation experiments; partial or total extirpation of the cartilage resulted in diminished facial growth in rabbits and in rats. However, these findings were challenged by Moss et al and Stenström and Thilander, who interpreted the altered midface after extirpation of the septal cartilage as a collapse of the nasal dorsum rather than as a reduction of anteroposterior growth. They concluded that the septal cartilage acts only as a supportive strut of the nasal bone with a passive role in growth. A recent study cast doubt on the role of the septum as a mechanical strut but did not elucidate the role of the septal cartilage in anteroposterior midfacial growth.
Anteroposterior midfacial growth is a complex combination of sutural displacement and bone apposition at the posterior surface of the maxillary tuberosity. Sutures, in particular, are major contributors to postnatal growth. However, it is generally agreed that sutures have no intrinsic growth potential ; they respond to forces that tense the tissue of the suture, resulting in bone formation at the sutural edges. Most studies support the idea that growth of internal soft tissues is a major source of such force. For example, growth of the brain probably tenses the calvarial sutures, and expansion of the eyeball might tense the orbital sutures. The nasofrontal suture in pigs is heavily compressed by mastication, yet it is rapidly growing. The source of the force that separates this suture has been an intellectual dilemma. Many authors believe that the expansion of the nasal septal cartilage stretches the midfacial sutures including the nasofrontal suture, whereas others believe that the growth at this suture and the enlargement of the nasal septal cartilage are adaptive and occur in response to functional demands, such as increased nasal airway size and expansion of the nasal capsule as described by the functional matrix theory.
In-vitro studies indicate that growth of the septal cartilage is not uniform. The proliferation rate of septal chondrocytes tends to be higher in the most posterior and anterior regions than in the middle. Such variations might be a cause of growth rotations of the midface; in experimental animals, regional differences might explain the variable outcomes of extirpation experiments.
Septal cartilage growth in vivo and in vitro is stimulated by growth hormones and growth factors. Growth hormone deficiency results in a decrease of the anteroposterior dimension of the cranial base, including the nasal septum. On the contrary, acromegaly (increased growth hormone) patients are characterized by increased facial dimensions attributed in part to the stimulation of septal growth. Response to growth hormones suggests that septal growth might be episodic, occurring in relation to the episodic secretion of growth hormones in mammals. If so, then a primary role for the cartilage in powering midfacial growth suggests that growth episodes in the nasal septum might precede, and would never lag behind, growth episodes in the nasofrontal suture.
The controversy around the active vs the passive role of the nasal septal cartilage in midfacial growth can be attributed, in part, to limitations associated with traditional experimental approaches. Organ culture growth is clearly not comparable with the in-vivo situation. The surgical extirpation model is associated with unavoidable trauma and variability in the extirpation location. In addition, both culture and extirpation studies suffer from the lack of direct comparison between the growth of the septal cartilage and that of the sutures it is thought to expand.
This study was undertaken to improve on these experimental approaches by making telemetric, continuous, and simultaneous measurements of the anteroposterior growth of the septal cartilage (at 2 sites) and the nasofrontal suture. Pigs were used as the study model because of the space requirements for instrumenting the nasal cavity and to allow comparisons with previous studies on nasofrontal suture growth and mechanical deformation of the septal cartilage in vivo and in vitro. This technique produced direct measurements of the interstitial growth rate of the septal cartilage. Furthermore, comparison of the 2 sites allowed a determination of whether septal growth is uniform. Comparing real-time anteroposterior growth of the septal cartilage and the nasofrontal suture was intended to test the hypothesis that septal growth leads to sutural growth. If growth of the septal cartilage drives midfacial growth, then it should precede that of the nasofrontal suture or exceed it in amount. Because the recording was continuous, it was possible to investigate whether growth was circadian or episodic, which would suggest possible associations with underlying systemic phenomena such as growth hormone levels.
Material and methods
The subjects for this study were 10 female Hanford minipigs ( Sus scrofa ), ranging in age from 3.5 to 4.5 months and weighing 12 to 27 kg ( Table I ). The pigs were obtained from Sinclair Research Farms (Columbia, Mo). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington.
| Animal ID | Age (mo) | Weight (kg) | Modifications from protocol | DVRT angle relative to the long axis of the nasal bone (°) | ||
|---|---|---|---|---|---|---|
| Anterior septal cartilage | Posterior septal cartilage | Nasofrontal suture | ||||
| 411 | 4 | 18 | 2 | 18 | 3 | |
| 412 | 3.5 | 14 | 1 window | 14 | – | – |
| 413 | 4.5 | 19 | 1 window | 6 | – | – |
| 417 | 4 | 12 | 1 window | 15 | 4 | 3 |
| 422 | 3.5 | 18 | 2 | 9 | 4 | |
| 427 | 4.5 | 18 | 4 | 2 | 1 | |
| 428 | 4.5 | 20 | 1 bone plate was not replaced | – | – | 1 |
| 429 | 4.5 | 27 | – | – | 6 | |
| 430 | 4.5 | 27 | 2 | 13 | 2 | |
| 431 | 4 | 20 | 10 | 8 | 4 | |
| Average ± SD | 4.2 ± 0.4 | 19.3 ± 4.8 | 7 ± 5 | 9 ± 6 | 3 ± 2 | |
Differential variable reluctance transducers (Microstrain, Williston, Vt) were used to measure growth at the septal cartilage and the nasofrontal suture. This linear displacement transducer comprises 2 parts, a rod and a coiled core ( Fig 1 ), each fixed in the tissue by a barb or a screw. The rod slides in the core as tissue length changes, and its position is detected by measuring the coil’s differential reluctance. This differential detection method provides a sensitive measure of rod movement (±1 μm). The linear displacement of the rod over time, standardized by initial length, was the measure of growth. While the pig was feeding, the displacement of the rod could also be used to assess functional deformation. Differential variable reluctance transducer signals were conditioned and amplified and then telemetered to a nearby computer with a USB base station (Microstrain), where the signals were captured at 1-minute intervals.
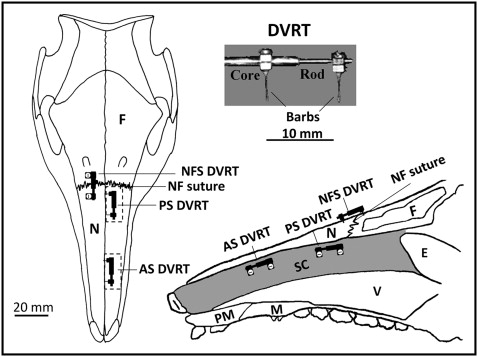
Instruments were implanted under aseptic conditions in a dedicated surgical suite. The pigs were premedicated by intramuscular injection of oxymorphone (0.1 mg/kg, analgesic), acepromazine (0.5 mg/kg, sedative), glycopyrrolate (0.01 mg/kg, antisialagogue), and buprenorphine (0.01 mg/kg, analgesic). The pigs were then mask anesthetized with isoflurane/nitrous oxide and intubated. After intubation, 2 mL of 2% lidocaine with 1:200,000 epinephrine was injected subcutaneously on the dorsal surface of the snout.
A 5-cm supraperiosteal midline incision was made to expose the dorsal surface of the nasal and frontal bones and the nasofrontal suture. An oscillating saw (Stryker, Kalamazoo, Mich) was used to cut two 2-cm windows in the left nasal bone ( Fig 1 ). The posterior window was positioned 5 mm in front of the nasofrontal suture, and the anterior window was about 2 cm more anterior. The lateral periosteum of each window was kept intact to ensure adequate blood supply. Bone was reflected to expose the underlying parietotectal cartilage. Electrosurgery was used to cut through the parietotectal cartilage to reach the nasal cavity. Bone wax and Gelfoam (Pfizer, New York, NY) were used when needed to control bleeding. In each window, a differential variable reluctance transducer was implanted horizontally in the dorsal half of the cartilaginous nasal septum ( Fig 1 ). The barbs traversed most or all of the thickness of the septum from left to right. On the intact right side, a 19-gauge needle was used to create holes in the nasal and frontal bones, and a third differential variable reluctance transducer was implanted spanning the right nasofrontal suture with screws rather than barbs. The distance between the barbs or screws of each differential variable reluctance transducer was measured and used to standardize the growth measurements. The bony windows were replaced and fixed using miniplates and screws (Stryker). The lead differential variable reluctance transducer wires were fixed to the surrounding tissues with silk sutures. A subcutaneous tunnel was made at the posterior limit of the incision to emerge at the back of the neck. A 2-mL osmotic pump (Alzet, Cupertino, Calif) loaded with antibiotics (amikacin, 250 mg/mL; or gentamicin, 40 mg/mL) was implanted near the exit site in the back of the neck. The incisions were sutured. A lateral cephalogram was taken to measure the orientation of the differential variable reluctance transducer to the long axis of the nasal bone. A fentanyl patch (25 or 50 μg/h) (Duragesic; ALZA, Mountain View, Calif) was then applied. The animals were allowed to awaken; after their recovery, buprenorphine was injected (0.01 mg/kg) to provide analgesia until the fentanyl took effect. Throughout the survival time after the surgery, the animals were constantly monitored for signs of infection and discomfort. Analgesics (buprenorphine) or antibiotics (trimethoprim or clavamox) were administered when needed.
The differential variable reluctance transducer wires were connected to a signal demodulator (DEMOD-DC2; Microstrain) attached to a 3-channel wireless transmitter (V-link; Microstrain) that broadcasted at a frequency of 915 MHz. The demodulator and transmitter were housed in a jacket worn by the animal and powered by a long-life 19-mA battery to ensure continuous recording of the differential variable reluctance transducers’ lengths. Data were collected every minute from the 3 locations via the USB base station receiver, connected to a computer running Agile-link software (Microstrain). Recordings continued for as long as at least 1 differential variable reluctance transducer was functioning correctly. Because of instrument failure, monitoring time and location varied. Table II summarizes the number of hours recorded from each location for all animals. When the instruments had stopped recording (usually because of breakage), the animals were anesthetized by isoflurane and killed by intracardiac perfusion or a lethal dose of pentobarbital in an ear vein. Then the head was dissected, and the differential variable reluctance transducers were visually inspected to check for positioning.
| Animal ID | Total hours of growth recorded | Number of 24-hour cycles | ||||
|---|---|---|---|---|---|---|
| Anterior septal cartilage | Posterior septal cartilage | Nasofrontal suture | Anterior septal cartilage | Posterior septal cartilage | Nasofrontal suture | |
| 411 | 110 | 120 | 160 | 3 | 2 | 0 |
| 412 | 23 | 0 | 0 | 0 | 0 | 0 |
| 413 | 30 | 0 | 0 | 1 | 0 | 0 |
| 417 | 127 | 99 | 165 | 3 | 3 | 3 |
| 422 | 47 | 37 | 47 | 1 | 0 | 1 |
| 427 | 86 | 50 | 48 | 0 | 1 | 1 |
| 428 | 0 | 0 | 40 | 0 | 0 | 1 |
| 429 | 20 | 0 | 80 | 0 | 0 | 2 |
| 430 | 272 | 80 | 272 | 11 | 3 | 7 |
| 431 | 40 | 67 | 53 | 1 | 2 | 2 |
The differential variable reluctance transducers’ voltage was converted to displacement using a calibration procedure before each experiment. The differential variable reluctance transducer lengths at the 3 locations were plotted against real time (as recorded by the computer clock). The difference between the initial (average of first 2 hours) and final (average of last 2 hours) differential variable reluctance transducer lengths was taken as the total growth at each location, and this was converted to growth strain by dividing by the initial differential variable reluctance transducer length in millimeters. Total growth strain was divided by total monitoring time (in hours) to calculate the growth strain per hour. Differential variable reluctance transducers measure linear displacement. Since the actual major axis of growth of the septum is unknown, the differential variable reluctance transducers’ measurements might have underestimated the true growth. Because a pig snout grows primarily in a horizontal (anteroposterior) direction, and the nasofrontal suture is restricted to growth along this axis, we intended to place all differential variable reluctance transducers parallel to long axis of the nasal bone (ie, the horizontal axis) to assess growth of the cartilage and the suture along the horizontal (anteroposterior) axis. However, perfect parallelism was surgically impossible. Even for the nasofrontal suture, the lateral cephalometric x-ray showed minor variations in the differential variable reluctance transducers’ angulations ( Table I ). Therefore, growth strains were calculated using both the total measured strain and the horizontal component of strain from each differential variable reluctance transducer. The horizontal (anteroposterior) component of growth strain was calculated as (growth strain per hour) × (cos ), where is the angle between the long axis of the differential variable reluctance transducer and the long axis of the nasal bone as determined from the lateral cephalometric x-ray ( Table I ). The total measured growth strain and the horizontal component of growth strain never differed by more than 5%, with identical statistical results. Thus, only the horizontal component is reported here to focus on the anteroposterior growth of the septal cartilage and the nasofrontal suture.
The existence of a regional (anterior vs posterior) difference in the growth of the septal cartilage was tested using paired t tests. Growth of the septal cartilage was compared with that of the nasofrontal suture to test the hypothesis that the septal cartilage grows faster than the nasofrontal suture using analysis of variance and paired t tests as appropriate. Statistics were analyzed with Excel (Microsoft, Redmond, Wash) and SPSS software (SPSS, Chicago, Ill).
The growth patterns of the septal cartilage and the nasofrontal suture were examined by plotting the differential variable reluctance transducer lengths against recording time in 24-hour increments using KaleidaGraph (Synergy Software, Reading, Pa). Since data were gathered at 1-minute intervals, each increment in the plot was 1/60 of an hour (approximately 0.01667 hour). Each plot started at 6:00 pm in real time (as indicated by the computer clock). In some cases, data acquisition was interrupted (eg, a differential variable reluctance transducer was accidentally unplugged overnight); thus, full-day data were not always available. However, in most animals, at least one 24-hour cycle in at least 1 location was recorded. Table II lists the available 24-hour data sets. A 10% weighting algorithm (locally weighted scatterplot smoothing; KaleidaGraph) was applied to the plots. This smoothing algorithm fits simple models to localized subsets of the data to build up a function that describes the variations in the data, point by point. The weighted data were used to calculate the derivative of growth (ie, growth speed). These growth speeds were used to compare the cartilage and the suture locations. In addition, these data were used to determine whether the growth pattern better fit an episodic or a continuous model. If growth is continuous, then little variation in growth speed should be observed, whereas episodic growth should show bursts. Bursts were arbitrarily defined as time intervals longer than 1 hour during which growth speed was at least 3 times the average growth speed over the total time of recording. The number and duration of bursts in the 24-hour periods and during the day (6:00 am -6:00 pm , when the lights were on in the animal housing facility) and night (6:00 pm- 6:00 am ) were counted and averaged. In addition, the number of “growing” hours (derivative >0) and “nongrowing” hours (derivative ≤0) were calculated. Values were compared to test whether there was any circadian variation in growth.
Cross-correlation analysis for time series (Statistica; StatSoft, Tulsa, Okla) was used to compare the timing of growth at the 3 sites. This analysis compares pairs of waveform plots in terms of their overall correlation and relative timing. First, correlations between raw growth curves at the 3 sites were calculated and compared for time lags ±150 minutes. This was intended to assess whether the growth of the cartilage preceded that of the suture. Second, similar correlations were calculated for the growth speed curves (derivative of the raw growth data) to test whether growth bursts of the septal cartilage preceded bursts at the nasofrontal suture.
Results
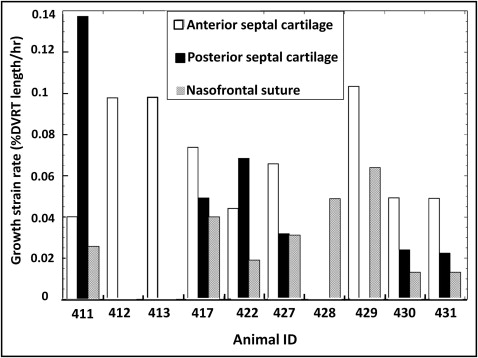
All locations showed growth as evidenced by increases in differential variable reluctance transducer length ( Table III ; Fig 2 , left ; Fig 3 ). The highest average horizontal growth strain rate was seen in the anterior cartilage (0.07% ± 0.03%/h; n = 9) followed by the posterior cartilage (0.06% ± 0.04%/h; n = 6) and nasofrontal suture (0.03% ± 0.02%/h; n = 8). The 2 cartilage locations did not differ, so cartilage values were averaged to allow comparison of the septal cartilage and nasofrontal suture. The cartilage was found to grow faster in the 7 animals with simultaneous readings from the nasofrontal suture and at least 1 cartilage site ( P = 0.002; paired t test) and in the total sample of 10 animals ( P = 0.004; 2-sample t test). Analysis of variance was used for a more detailed examination of the 3 locations; the marginally significant result ( P = 0.05) was found by post-hoc testing to be due to the higher growth rate of the anterior septal cartilage compared with the nasofrontal suture ( P = 0.04).
| Animal ID | Anterior cartilage growth strain rate | Posterior cartilage growth strain rate | Averaged cartilage growth strain rate | Nasofrontal suture growth strain rate |
|---|---|---|---|---|
| 411 | 0.04 | 0.13 | 0.09 | 0.03 |
| 412 | 0.11 | NA | 0.10 | NA |
| 413 | 0.10 | NA | 0.10 | NA |
| 417 | 0.07 | 0.05 | 0.06 | 0.04 |
| 422 | 0.04 | 0.07 | 0.06 | 0.02 |
| 427 | 0.07 | 0.03 | 0.05 | 0.03 |
| 428 | NA | NA | NA | 0.05 |
| 429 | 0.10 | NA | 0.10 | 0.06 |
| 430 | 0.05 | 0.02 | 0.03 | 0.01 |
| 431 | 0.05 | 0.02 | 0.04 | 0.01 |
| Average ± SD | 0.07 ± 0.03 † | 0.06 ± 0.04 | 0.07 ± 0.03 ‡ | 0.03 ± 0.02 † ‡ |
∗ Measured growth strain and the horizontal component of growth strain gave identical averages and statistical results.
† the anterior septal cartilage grew significantly faster than the nasofrontal suture ( P <0.001; paired t test).
‡ the averaged cartilage growth strain rate was significantly higher than the nasofrontal suture ( P = 0.002; paired t test).
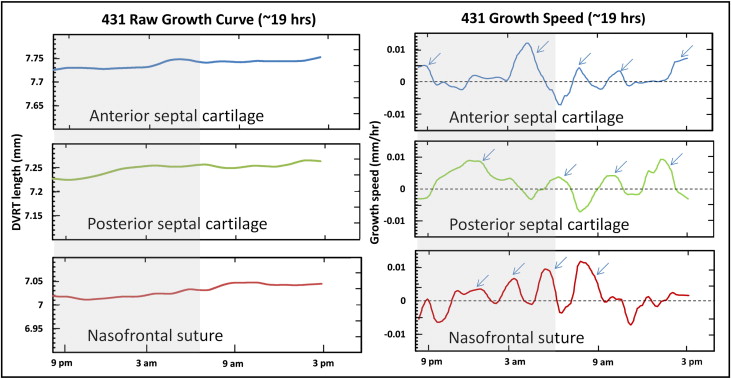
The pattern of growth, as shown by the derivative function of the growth curve, was episodic at all locations with alternating periods of active growth bursts and periods of no growth or shrinkage ( Fig 2 , right ). The duration, amplitude, and number of peaks are listed in Table IV . The total duration of active growth (growth speed >0) ranged from 55% to 60% with about 5 bursts per 24-hour cycle, each lasting about 3 hours. Stasis and shrinkage each used about 20% of the total time. The amplitude of growth bursts was 5- to 10-fold higher than the average growth rate per hour. The 3 locations were statistically indistinguishable in all growth-burst parameters. Although the periodicity of the bursts was clear, there was substantial variability among pigs and among locations within pigs. All pigs and locations sampled showed growth bursts in the early morning (about 2:00-6:00 am ), late morning (about 8:00-11:00 am ), and evening (4:00-7:00 pm ); the other bursts were irregular ( Table V ).
| Location | Peaks in 24 hours (n) | Peak duration (h) | Amplitude relative to the averaged growth speed (fold difference) | Growth hours of a 24-hour cycle (%) | Nongrowing hours of a 24-hour cycle (%) | Negative growing hours of a 24-hour cycle (%) |
|---|---|---|---|---|---|---|
| Anterior septal cartilage (n = 6) | 5.6 ± 0.6 | 2.6 ± 0.4 | 10 times ± 5 | 55 ± 17 | 21 ± 5 | 23 ± 8 |
| Posterior septal cartilage (n = 5) | 4.6 ± 1.5 | 3.1 ± 0.8 | 5 times ± 3 | 57 ± 33 | 21 ± 6 | 21 ± 7 |
| Nasofrontal suture (n = 7) | 4.8 ± 0.7 | 3.0 ± 0.7 | 7 times ± 5 | 61 ± 27 | 20 ± 6 | 18 ± 6 |
| Animal ID | Evening | Around midnight | After midnight | Morning | Early afternoon | Late afternoon |
|---|---|---|---|---|---|---|
| Anterior septal DVRT | ||||||
| 411 | 4:00 pm -8:30 pm | 10:00 pm -12:30 am | 2:00 am -6:00 am | 8:00 am -10:00 am | – | 4:00 pm -6:00 pm |
| 417 | 5:00 pm -7:30 pm | 11:30 pm -1:30 am | 4:00 am -7:00 am | 8:00 am -11:30 am | 3:00 pm -4:00 pm | 5:00 pm -6:30 pm |
| 422 | 4:30 pm -8:00 pm | 10:00 pm -1:00 am | 2:30 am -4:20 am | 7:30 am -9:00 am | 11:00 am -2:30 pm | 4:00 pm -6:00 pm |
| 430 | 4:00 pm -7:30 pm | 9:30 pm -12:30 am | 4:00 am -5:40 am | 9:00 am -10:30 am | 12:30 pm -2:30 pm | 4:00 pm -6:00 pm |
| 431 | 5:00 pm -10:00 pm | 12:30 am -2:30 am | 3:00 am -5:30 am | 7:30 am -8:30 am | 1:00 pm -4:30 pm | – |
| Posterior septal DVRT | ||||||
| 411 | 10:00 pm -2:00 am | – | 3:00 am -4:30 am | 8:30 am -11:00 am | 12:00 noon-3:00 pm | – |
| 417 | 10:00 pm -12:00 am | 1:30 am -4:00 am | 6:00 am -9:00 am | 9:30 am -11:00 am | 12:30 pm -3:00 pm | 4:00 pm -6:00 pm |
| 427 | 4:00 pm -10:30 pm | – | 3:00 am -6:00 am | 6:30 am -10:00 am | 1:00 pm -3:00 pm | 4:00 pm -6:00 pm |
| 430 | 5:00 pm -7:00 pm | 10:00 pm -1:00 am | 1:30 am -4:00 am | 5:00 am -9:00 am | 10:00 am -2:00 pm | – |
| 431 | – | 10:00 pm -2:00 am | 3:30 am -5:30 am | 7:30 am -9:30 am | 10:30 am -12:30 pm | – |
| Nasofrontal suture DVRT | ||||||
| 417 | 8:00 pm -10:30 pm | 11:00 pm -2:00 am | 2:30 am -4:00 am | 5:00 am -6:00 am | 7:00 am -10:00 am | 11:30 am -1:00 pm |
| 422 | 4:00 pm -11:00 pm | 1:30 am -3:00 am | 5:00 am -6:30 am | 8:30 am -11:00 am | – | 1:00 pm -6:00 pm |
| 427 | 4:00 pm -12:00 am | – | 5:00 am -6:30 am | 9:00 am -10:30 am | 11:30 am -1:00 pm | 1:30 pm -3:00 pm |
| 428 | 4:00 pm -8:30 pm | 10:30 pm -1:00 am | 2:30 am -8:00 am | 8:30 am -11:00 am | 12:00 noon-2:00 pm | – |
| 429 | 4:00 pm -11:00 pm | 12:00 midnight-3:00 am | 3:30 am -6:00 am | 9:00 am -2:00 pm | – | – |
| 430 | 5:00 pm -8:00 pm | 8:30 pm -10:30 pm | 11:30 pm -1:00 am | 2:00 am -5:30 am | 7:30 am -10:00 am | – |
| 431 | 8:00 pm -9:30 pm | 1:00 am -3:00 am | 4:00 am -5:30 am | 6:00 am -7:00 am | 11:30 am -2:30 pm | – |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


