Treatment of the Cervical Nodes
Results of Local and Locoregional Radiotherapy
Determination of the Radiation Fields
Conformal versus Conventional Radiotherapy Techniques
Introduction
Salivary gland malignancies are traditionally treated with surgery, with postoperative radiotherapy if there are unfavorable prognostic factors for locoregional recurrence.1 In the past, salivary gland tumors were considered to be resistant to radiotherapy.2 However, even in patients with medically and/or technically inoperable disease, primary radiotherapy can be regarded as an option for curative treatment. In addition to conventional photon and electron therapy, particle therapy as a primary treatment for malignant salivary gland tumors may enhance the locoregional cure rates in advanced cases. This chapter discusses prognostic factors, with special emphasis on the value of the new histological subclassification, comorbidity, and indications for primary and postoperative radiotherapy for malignant salivary gland tumors. Several radiation techniques are outlined.
Patient Selection
 Prognostic Factors
Prognostic Factors
The decision on whether to proceed with postoperative radiotherapy after curative surgery for salivary gland cancer depends on the prognostic factors in the individual patient. As radiotherapy targets possible local and regional tumor extension, the prognostic factors focus on locoregional tumor control. Several factors are discussed in detail in Chapters 25, 29, and 35.
To summarize the pretreatment factors, the site of the primary tumor is a prognostic factor for local control, favoring tumors of the oral cavity.3,4 Salivary gland tumors in the submandibular glands have the highest risk of positive lymph nodes, up to 42%.3 The clinical T stage and N stage are prognostic factors for locoregional control, independent of site.3–5 Impairment of the function of the facial nerve at presentation is a known prognostic factor for local and regional control.6,7 Location of the tumor in the medial parotid lobe is associated with an increased risk for facial nerve involvement.6 Impaired facial nerve function correlates with symptoms of pain and with skin and perineural invasion.6,7 Some studies have reported that more advanced age is a negative prognostic factor for locoregional control.8,9 In a study by Vander Poorten et al., pain and skin invasion were reported to be prognostic for disease-free survival in patients with parotid gland tumors.7
Independent histological prognostic factors derived from the resection specimen are close or incomplete resection margins, bone invasion, perineural growth, size, and pN status.3
 Postoperative radiotherapy is indicated on the basis of negative prognostic factors for locoregional control: close or incomplete resection margins, bone invasion, perineural growth, facial nerve invasion, large size (pT3/4), and advanced pN.
Postoperative radiotherapy is indicated on the basis of negative prognostic factors for locoregional control: close or incomplete resection margins, bone invasion, perineural growth, facial nerve invasion, large size (pT3/4), and advanced pN.
 Histological Classification
Histological Classification
Histological type was not found to be a prognostic factor in parotid gland cancer in the study by Vander Poorten et al. and was therefore not included in the prognostic score.7 In some publications, tumors are grouped into high-grade and low-grade categories. The low-grade category includes mucoepidermoid and acinic cell carcinoma, and sometimes adenoid cystic carcinoma.7,10,11 Grading was an independent prognostic factor for disease-free survival in some studies,10,11 but not all.7 This difference may be caused by the poor interobserver reproducibility for grading.12
In 1972, the World Health Organization (WHO) subdivided salivary gland malignant tumors into six subcategories (see Chapter 27 for more details on the classification of malignant salivary gland tumors), with a category of adenocarcinoma, not otherwise specified (NOS). The latter category was subdivided into 10 subcategories in the WHO 1991 classification.13 A new WHO classification was recently published including four extra subtypes.14 The histological subtype (WHO 1972) may be predictive of distant metastases,3 but not of locoregional control.3,10,15–17 A major reason for this may be the high percentage of patients with poor prognostic factors who receive postoperative radiotherapy, probably improving locoregional control.
Of the 10 new subcategories in the WHO 1991 classification, only three were diagnosed more frequently in a large Dutch retrospective study. All pathology slices from patients diagnosed with adenocarcinoma NOS were reevaluated by three experienced pathologists. Forty salivary duct carcinomas, 34 polymorphic low-grade carcinomas, and 14 epithelial–myoepithelial carcinomas were diagnosed in a total of 666 salivary gland carcinomas. Other subtypes such as basal cell adenocarcinoma (n = 3), papillary cystadenocarcinoma (n = 1), mucinous adenocarcinoma (n = 1), oncocytic carcinoma (n = 1), and malignant myoepithelial carcinoma2 were rarely diagnosed. Sebaceous carcinoma was not encountered in this series.
Salivary Duct Carcinoma
Salivary duct carcinoma is a high-grade tumor, predominantly seen in male patients and located in the parotid gland. It resembles ductal carcinoma of the breast. These lesions are frequently positive for androgen receptor, HER-2/neu, and p53.14,18 Expression of HER-2/neu and p53 is associated with early recurrences, distant metastases, and poor survival.18 The 5-year disease-free survival in the Dutch study was only 14%, with a cumulative 5-year risk for local and regional recurrence of 30% and for distant metastases of 80%.
The aggressiveness of this tumor justifies, in addition to extended surgery even in patients with T1 lesions, ipsilateral functional neck dissection and postoperative radiotherapy.18,19 Brandwein-Gensler et al. reported on a series of 16 patients, 15 of whom had tumors in the parotid gland representing a low-grade salivary carcinoma with a primarily in situ intraductal process. All of the patients who underwent surgery had a disease-free survival.20 However, low-grade salivary duct carcinoma might possibly be better classified as low-grade cribriform cystadenocarcinoma.14
Polymorphous Low-Grade Adenocarcinoma
Polymorphous low-grade adenocarcinoma is predominantly seen in female patients and located in the minor salivary glands of the palate. In the Dutch study, no distant metastases were seen in 34 patients, and the disease-free 5-year survival was 84%; nine of the 34 patients were treated with postoperative radiotherapy. Castle et al. published a large series including 164 patients with polymorphous low-grade adenocarcinoma with a long follow-up period.21 Conservative complete surgical excision was the treatment of choice, with postoperative radiotherapy in only 10% of the patients. The recurrence rate was only 9%, and recurrences were more frequently seen in tumors of the hard palate. In a series of 40 patients, Evans et al. compared the local recurrence rates in patients with polymorphous low-grade adenocarcinoma and positive or unknown surgical margins.22 No recurrences were seen in nine patients who received postoperative radiotherapy, in comparison with 10 recurrences among 13 after who received surgery alone.
Epithelial–Myoepithelial Carcinoma
Epithelial–myoepithelial carcinoma is predominantly found in the parotid gland, with an equal male/female distribution. All 14 patients in the Dutch study remained free of locoregional recurrences, and 11 received postoperative radiotherapy. Distant metastases were seen in only one patient, indicating low-grade disease. Savera et al. analyzed 25 patients exclusively with myoepithelial differentiated carcinoma. Ten of the lesions were cytologically high-grade and 15 were low-grade. Only six of the patients received postoperative radiotherapy. Distant metastases and locoregional recurrences were seen in almost 50% of the cases. The authors noted that the diagnostic criteria for myoepithelial carcinoma may be confusing, making it difficult to distinguish from malignant mixed tumors and epithelial–myoepithelial carcinoma.23
Basal Cell Adenocarcinoma
Basal cell adenocarcinoma is typically located in the hard palate, rarely with metastases to the cervical neck nodes. This is a low-grade tumor, but local recurrences are frequently seen. Postoperative radiotherapy is indicated when the resection margins are incomplete or close.24
Malignant Oncocytoma
Malignant oncocytoma is mainly located in the parotid gland. In a review of the literature including 36 cases, the local recurrence rate with surgery alone was 40%, and a combination with postoperative radiotherapy significantly improved local control.25
Papillary Cystadenocarcinoma
A review of 57 patients with papillary cystadenocarcinoma noted that this type of tumor shows indolent biological behavior. Most of the tumors were located in the parotid gland, and one-third were in the minor salivary glands of the oral cavity.26 Most papillary cystadenocarcinomas are therefore low-grade, although very late distant metastases have been reported.
Mucinous Adenocarcinoma
Mucinous adenocarcinoma is a rare, high-grade malignancy, mainly found in the palate and floor of mouth, with a high risk for positive cervical neck nodes and distant metastases.27
Sebaceous Carcinoma
Sebaceous carcinoma is also a very rare disease, mostly located in the parotid gland; it is slow-growing and is considered to be low-grade.28
In conclusion, several new histological subcategories of salivary gland carcinomas have been described. Although the prognosis varies considerably between these subtypes, the histological typing can be very difficult, even for experienced pathologists. In most studies, histological subtype is not an independent predictive factor for locoregional control; other pathological parameters, such as the status of the resection margins, bone invasion, and perineural invasion are more important.
 Decision-making for postoperative radiotherapy on the basis of the histological subclassification is difficult, as the histological typing of these tumors is difficult. Accordingly, studies on the prognostic impact of the histological subtype tend to be controversial.
Decision-making for postoperative radiotherapy on the basis of the histological subclassification is difficult, as the histological typing of these tumors is difficult. Accordingly, studies on the prognostic impact of the histological subtype tend to be controversial.
 Comorbidity
Comorbidity
In contrast to other types of head and neck cancer, smoking and alcohol abuse are not associated with salivary gland malignancies. Tobacco and alcohol consumption is associated with pulmonary, cardiovascular, hepatic, and metabolic diseases. Comorbidity is therefore less frequently seen in salivary gland cancer in comparison with squamous cell carcinoma of the head and neck.29 In some studies on the treatment of head and neck cancer, comorbidity is an independent prognostic factor for recurrences.30
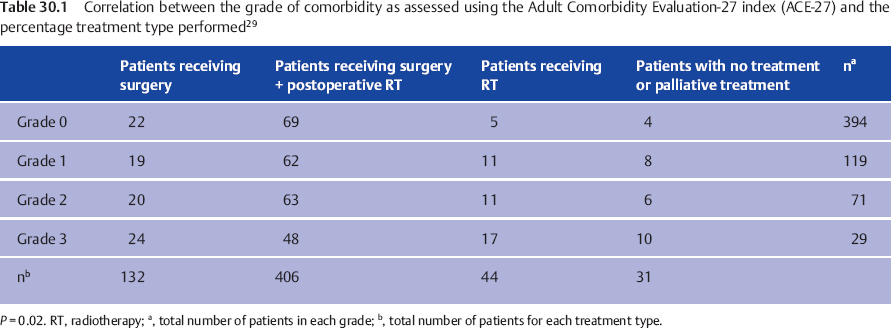
Comorbidity can be scored using the Adult Comorbidity Evaluation-27 index (ACE-27). This is a validated 27-item list, graded into four levels ranging from no comorbidity (0) to mild (grade 1), moderate (grade 2), and severe (grade 3). Comorbidity, particularly grades 2 and 3, is a predictive factor for major complications after head and neck surgery, overall survival, and, in some studies, for disease-free survival.31
The role of comorbidity in patients with salivary gland cancers was studied in a group of 613 patients in the Netherlands.29 The distributions of ACE-27 grades 0–4 were 64%, 19%, 12%, and 5%, respectively. Interestingly, the distribution of ACE-27 grades was equal for all histological subcategories except for squamous cell carcinoma in the salivary glands. Grades 1–3 were seen in two-thirds of patients with squamous cell carcinoma, compared with one-third for all other histological subcategories. In this study, comorbidity significantly influenced the overall survival, but not the disease-free survival.29 Comorbidity only played an important role in the choice of treatment in patients with grade 3; patients with grade 3 comorbidities received primary radiotherapy significantly more often in comparison with those with grades 0–2. On the other hand, postoperative radiotherapy was administered significantly less in those with grade 3 comorbidity (Table 30.1). In general, therefore, comorbidity should not influence the choice of treatment unless a patient has severe comorbidities.
 Age
Age
Age has also been shown to be an important determinant in the choice of treatment. Derks et al. showed that patients over the age of 70 with advanced head and neck cancer received nonstandard treatment significantly more often in comparison with younger patients.32
 In contrast to patients with other types of head and neck cancers, comorbidity in salivary gland cancer patients is low and has less impact on decision-making for adjuvant therapy. In general, however, the only important factor involved in changing the standard treatment is comorbidity.
In contrast to patients with other types of head and neck cancers, comorbidity in salivary gland cancer patients is low and has less impact on decision-making for adjuvant therapy. In general, however, the only important factor involved in changing the standard treatment is comorbidity.
Indications for Radiotherapy
 Local Treatment
Local Treatment
Primary treatment of salivary gland carcinoma consists of surgery. In general, primary radiotherapy is reserved for patients who are unfit for surgery (ACE-27 grade 3), patients with inoperable disease, and patients with palliative cases who present with distant metastases. Indications for postoperative radiotherapy have been defined on the basis of negative prognostic factors for local control. The indications are close (< 5 mm) or incomplete resection margins, pathologically confirmed bone or perineural invasion, T3–T4 tumors, and recurrent disease.4,33–35 As mentioned earlier, a high-grade tumor is a relative indication.
 Treatment of the Cervical Nodes
Treatment of the Cervical Nodes
Neck dissection is the treatment of choice in patients with clinically positive cervical nodes. The indications for postoperative radiotherapy for the cervical nodes are the presence of positive nodes in the neck dissection specimen, close or incomplete margins, and complete paralysis of the facial nerve.3,6 In the Dutch study, independent prognostic factors for the presence of positive nodes were found to be T stage (T1 15%, T2 26%, T3 33%, and T4 33%), site of the primary tumor (oral cavity 9%, parotid gland 25%, submandibular gland 42%, pharynx/larynx 36%), and histology. Acinic cell carcinoma, adenoid cystic carcinoma, and carcinoma ex pleomorphic adenoma are low-risk, mucoepidermoid carcinoma is intermediate-risk, and squamous cell carcinoma and undifferentiated carcinoma are high-risk.
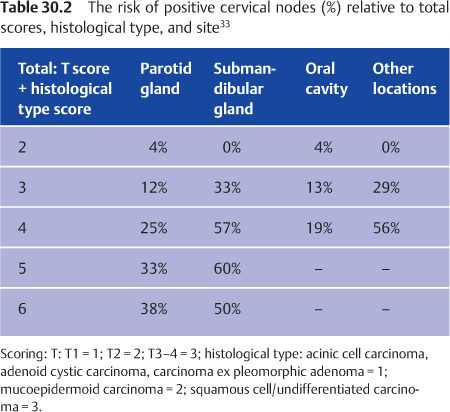
The need for elective treatment of the cervical nodes can be established using a rating scale based on site, T status, and histological type (Table 30.2). Elective neck treatment is indicated when there is a risk of more than 15%–20%. Elective treatment of the neck is therefore recommended in patients with parotid and oral cavity tumors with a score of 4 or more, and in patients with submandibular and pharynx/larynx tumors with a score of 3 or more.33 In accordance with Table 30.2, elective treatment of the neck is therefore indicated for all cases of submandibular cancer except T1 acinic cell carcinoma, adenoid cystic carcinoma, or carcinoma ex pleomorphic adenoma. Elective treatment of the neck is rarely indicated in patients with salivary gland tumors of the oral cavity. In addition to T stage and histology, high-grade lesions and facial nerve palsy have also been associated with a high percentage of occult lymph-node metastases.36,37
Levels to Treat
No elective treatment of the contralateral neck is generally indicated. In elective treatment of the neck for salivary gland cancer of the parotid glands, the periparotid nodes—at levels Ib, II, III and IV—should be treated. Levels I to III should be treated electively for salivary gland cancer of the submandibular glands.33,36,38 In a patient with a node-positive neck, levels I to V should be treated.33 The choice of neck node levels in elective treatment for oral cavity and laryngeal/pharyngeal salivary gland cancer is comparable with the general indications for squamous cell cancer in the same site.1
 Indications for postoperative neck radiotherapy:
Indications for postoperative neck radiotherapy:
 Curative neck radiotherapy: presence of positive nodes in the neck dissection specimen, close or incomplete margins, and complete paralysis of the facial nerve.
Curative neck radiotherapy: presence of positive nodes in the neck dissection specimen, close or incomplete margins, and complete paralysis of the facial nerve.
 Elective neck radiotherapy: if the risk is higher than 15%–20% (Table 30.2).
Elective neck radiotherapy: if the risk is higher than 15%–20% (Table 30.2).
 No elective treatment of the contralateral neck is indicated.
No elective treatment of the contralateral neck is indicated.
 Site-Specific Indications
Site-Specific Indications
The main added prognostic factor in parotid gland cancer is facial nerve palsy.6,17,34 Pretreatment facial nerve dysfunction is an independent prognostic factor for neck node recurrences,37 with a relative risk of 1.7, and 2.5 for partial and complete paralysis versus normal facial function, respectively.6 Lloyd et al. examined 2667 minor salivary gland cancers from the Surveillance Epidemiology and End Results (SEER) database. Neck node involvement was seen in 16%. Prognostic factors were male sex, stage T3–T4, pharyngeal site of the primary tumor, and high-grade adenocarcinoma or high-grade mucoepidermoid carcinoma.39
Another prognostically unfavorable factor is involvement of the skull base, as seen in some adenoid cystic carcinomas of the minor salivary gland.15 Postoperative radiotherapy, or primary radiotherapy in inoperable cases, is indicated.
Results of Local and Locoregional Radiotherapy
 Postoperative Radiotherapy
Postoperative Radiotherapy
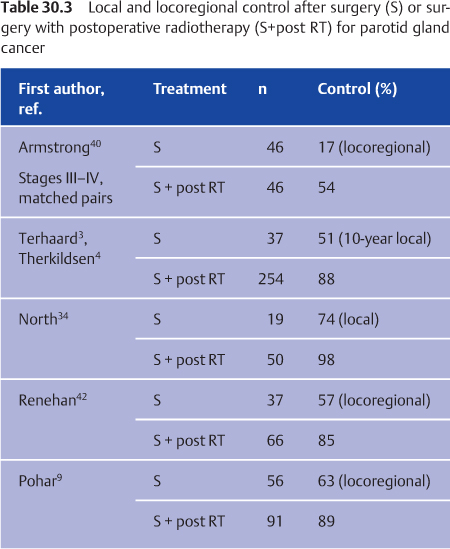
There have been no randomized controlled trials comparing surgery alone with surgery combined with postoperative radiotherapy. Armstrong et al. compared the two treatment options for stage III and IV major salivary gland cancers in a matched-pair analysis.40 Combined treatment significantly impaired survival. Improved survival with combined treatment has only been shown in a few retrospective studies.34,41 Prevention of locoregional recurrences is the major goal of postoperative radiotherapy. The role of postoperative radiotherapy as a prognostic factor for locoregional control has been extensively analyzed in some large studies. Postoperative radiotherapy is a strong prognostic factor for locoregional control (Table 30.3).3,4,9,16,40,42
In the nationwide Dutch study, combined surgery and postoperative radiotherapy were generally administered for patients with negative prognostic factors. Despite this negative selection, postoperative radiotherapy resulted in significantly higher 10-year local control rates in comparison with surgery alone for patients with T3–T4 lesions (84% vs. 18%), close resection margins (95% vs. 55%) or incomplete resection margins (82% vs. 44%), bone invasion (86% vs. 54%), and perineural invasion (88% vs. 60%).33 However, for patients with none of these prognostic factors, surgery alone resulted in 90% local control, and combined treatment is not recommended. The relative risk for local recurrence with surgery alone, in comparison with combined therapy, was 9.7.3,33
 There is a lack of prospective controlled studies, but on the basis of retrospective data, patients with salivary gland cancer and negative risk factors appear to benefit significantly from postoperative radiotherapy.
There is a lack of prospective controlled studies, but on the basis of retrospective data, patients with salivary gland cancer and negative risk factors appear to benefit significantly from postoperative radiotherapy.
< div class='tao-gold-member'>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses