http://evolve.elsevier.com/Hatrick/materials.
Acrylic resins are polymers that play an important role in removable prosthetics. The resins are used in the construction of complete and partial dentures, as well as maxillofacial prostheses (used to replace missing oral or facial structures). They can be used to simulate the oral mucosa, gingiva, and teeth. They are also used to reline these prostheses to improve their fit and to repair them when they break. Even though a commercial dental laboratory fabricates most of the removable prostheses, the allied oral health practitioner needs to be familiar with the materials and their uses, care, and repair. She or he must understand the materials used in prosthetic dentistry to better care for the patient and to assist the dentist. Patients who wear removable prostheses will often ask the dental assistant or hygienist questions that relate to the fit or home care of their prosthesis. This chapter covers the uses of acrylics for fabrication of complete and partial dentures and for relining, tissue conditioning, and repairing these prostheses. It also covers the use of acrylic denture teeth, porcelain denture teeth, and acrylic repair techniques. The instructions to be given to the patient for the care of these prostheses are presented.
Review of Polymer Formation
Polymers are large, long-chain molecules formed by chemically joining together smaller molecules, called monomers. The polymer chains will vary in length as the monomer is being consumed and may contain from 10,000 to 100,000 monomer units. The chains will lengthen until no more monomer is available. Most of the polymers shrink as they form, and this creates undesirable features in the final product.
Copolymers
When two or more different types of monomers join together, the polymer formed from them is called a copolymer. Copolymers are produced to enhance the physical and mechanical properties of the material. They are used in dentures to make them more resistant to fracture, in soft reline materials to make them soft and pliable, and in mouth guards to improve their shock-absorbing capacity.
Polymerization
The act of forming polymers is called polymerization. In general, less than 100% of the monomer is used up. The remaining unused monomer is called the residual monomer. The best clinical results occur when there is little residual monomer.
Cross-Linked Polymers
The polymer chains often have short chains of atoms attached to their sides. When the side chains of adjacent polymers bond together, the polymers are termed cross-linked polymers (see Figure 6-4 in Chapter 6 [Composites, Glass Ionomers, and Compomers]). When side chains of adjacent polymers are joined by weak bonds, the polymers are easily manipulated, bent, or stretched. When adjacent polymers are joined by highly charged side chains, the bond is stronger, and the cross-linked polymers are stronger and stiffer. They also are more wear resistant and, consequently, can be used in denture teeth. They polish more easily and are less affected by solvents such as alcohol.
Polymerization Reactions
There are two types of polymerization:
• Addition polymerization
• Condensation polymerization
The reactions are the same as for the impression polymers, addition silicones, and condensation silicones (see Chapter 15 [Impression Materials]).
Addition Polymerization
Addition polymerization is the most common form of polymerization for dental materials. It occurs in three stages:
Stage 1: Initiation (or induction)
Stage 2: Propagation
Stage 3: Termination
Unlike condensation polymerization, the reaction does not produce any by-products. Monomers have a core unit of two carbon atoms joined by a double bond. One carbon atom has two hydrogen atoms attached, and the other carbon atom has attached to it one hydrogen atom and one reactive group called a free radical. The free radical is made reactive by the chemical reaction of organic peroxide, such as benzoyl peroxide, with an activator or accelerator, such as a tertiary amine, or by heating.
Initiation
The free radical initiates the reaction by opening the bond between the two carbon atoms of the monomer. The broken carbon bond causes the monomer molecule to bond to another monomer. Each linkage leaves a free radical available for further reaction.
Propagation
The process of linking monomer units is termed propagation, and it continues until the monomer units are used up, or until a substance reacts with the free radical to tie it up.
Termination
When the free radical is tied up or destroyed, the process is terminated.
Curing Methods
The materials that react by chemical means are called chemical-curing, self-curing, or autopolymerizing. Materials that use heat to initiate the reaction are called heat-curing polymers. Materials in which the reaction is activated by light are called photo- or light-cured. Whether initiated by chemical means, light, or heat, the polymerization process releases heat (i.e., it is an exothermic reaction). The heat must be controlled during the process. If the temperature becomes too great, the monomer will vaporize and produce porosity in the material. Porosity weakens the material, causes it to discolor as stains are absorbed into the pores, and can lead to retention and growth of oral microorganisms and development of an unpleasant odor (“denture breath”).
Condensation Polymerization
Materials formed by a condensation reaction do not have many uses in dentistry. The condensation silicone impression materials are the most commonly known, and even they are not used much today. Typically, more than one type of monomer is used. The reaction itself produces by-products such as water, hydrogen gas, or alcohol that may compromise the physical properties or handling characteristics.
Acrylic Resins (Plastics)
Synthetic polymers used in prosthetic dentistry are called acrylic resins, because they are derived from acrylic acid. Acrylic resin forms when a liquid monomer (commonly methyl methacrylate) is mixed with a powder of small polymer beads, and the mixture undergoes polymerization. The polymerized resin is poly(methyl methacrylate) (PMMA). It is composed of numerous methyl methacrylate (MMA) monomer units linked together to form a long-chain polymer.
Uses
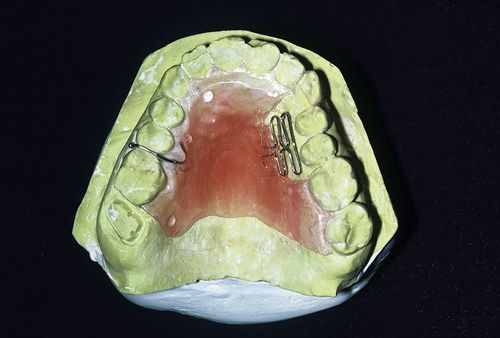
The resins are used for denture bases, denture teeth, relining and repair of prostheses, provisional acrylic partial dentures (flippers or stayplates), tissue conditioners, and custom impression trays. They also have uses as orthodontic retainers and removable tooth movement devices (Figure 17-1), bruxism mouth guards facings on esthetic crowns, and provisional restorations (see Chapter 18 [Provisional Restorations]). Specialized acrylic resins are used in esthetic tissue replacement for severe gingival recession and for facial reconstruction due to trauma, surgery, or birth defects. Acrylic resins are especially useful because they can be shaped to any contour and custom-colored to match the shade of the teeth, gingiva, or skin.
Modifiers
Acrylic resins used in dentistry are often modified by the addition of plasticizers, rubbers, and fillers to change their physical and mechanical properties. Plasticizers are liquids added to soften the acrylic plastics and make them more pliable. Oily liquids called aromatic esters are often used as plasticizers. Rubbers may be added to increase the impact fracture resistance of the acrylic resin. Fillers are added to strengthen the resin or change its optical properties. Chemical coupling agents may be used to bond the filler to the acrylic resin. This coupling prevents the filler particles from being plucked out of the resin by abrasion, the result of chewing on food, and makes the resin more wear resistant (similar to the use of silane to couple fillers in composite resins; see Chapter 6). These bonded fillers can be found in light-cured denture repair or impression tray materials.
Properties
Polymerization Shrinkage
Polymers undergo shrinkage as a result of the polymerization process. Heat-cured acrylic resins shrink about 6% by volume and about 0.2% to 0.5% linearly (from one point on the denture to another).
Dimensional Change
Sources of dimensional change in addition to polymerization shrinkage include water sorption and thermal expansion. A denture base will increase slightly in its overall size when it absorbs water. This expansion may help offset some of the shrinkage that occurs during polymerization. The coefficient of thermal expansion is more than twice that of composite resins (see Chapter 6).
Strength
The strength of the acrylic resins is fairly low, with a compressive strength of approximately 11,000 pounds per square inch (psi; or 75.8 megapascals [MPa]) and a tensile strength of 8000 psi (55.2 MPa). By comparison, amalgam has a compressive strength of about 60,000 psi (413.7 MPa). Acrylics are not very hard materials (Knoop hardness number, 16 to 18) and as a consequence are not very wear resistant. Although they have fairly good resistance to fatigue failure (can be flexed repeatedly before they break), they will break if dropped on the floor or in an empty sink during cleaning. To combat the brittleness and breakage problem, some manufacturers add butadiene-styrene rubber to the MMA to create a high-impact acrylic resin. An example of a high-impact acrylic resin is Lucitone 199 (Dentsply International), and it comes in four gingival shades.
Thermal Conductivity
Denture bases do not conduct temperature well. Patients wearing dentures will notice a marked difference when they eat foods such as ice cream or drink hot beverages. Because the denture partially insulates against the temperature of the food or beverage, patients may burn themselves when they attempt to swallow foods that are too hot.
Chemical-Cured versus Heat-Cured Acrylics
The type of processing has some effect on the properties. Polymerization is never 100% complete, and varying amounts of free monomer may be present in the polymerized material. In general, chemical-cured acrylic resins are weaker, softer, more porous, and less color stable than the heat-cured acrylic resins. After polymerization, there is more residual monomer in the chemical-cured acrylic (up to 5%), and it initially adversely affects many of the physical and mechanical properties until the monomer leaches out in minute amounts over several days or weeks. The tissues of some patients react to the residual monomer and become inflamed. Some dimensional change can occur during the first 24 hours in chemical-cured acrylic. For this reason, custom acrylic trays should not be used immediately, but should sit for 12 to 24 hours to allow most of the dimensional change to occur, so the impression will not be distorted. Heat-cured acrylics are harder, stronger, and less porous and have less than 1% residual monomer that leaches out of the surface relatively quickly. Properties of heat-cured acrylic resins are summarized in Table 17-1.
TABLE 17-1
Properties of Heat-Cured Acrylic Resins (PMMA)
| Property | Value |
| Polymerization shrinkage (by volume) | 6% |
| Polymerization shrinkage (linear) | 0.2%-0.5% |
| Coefficient of thermal expansion | More than twice that of composite |
| Compressive strength | 75.8 MPa (11,000 psi) |
| Tensile strength | 55.2 MPa (8000 psi) |
| Hardness (Knoop) | 15-18 kg/mm2 |
| Biocompatibility | Good |
| Thermal conductivity | Poor |
| Wear resistance | Fair |
| Fatigue resistance (to flexing) | Good |
| Impact resistance (to breakage when dropped) | Poor |
Porosity
Porosity in polymerized acrylic resin is characterized by the presence of many small or microscopic voids or pores. Porosity in the acrylic weakens it and makes it prone to collect debris and microorganisms. Denture odor and stains develop more readily. Porosity is a result of loss of monomer or inadequate pressure during processing. The monomer is highly volatile and can evaporate rapidly at room temperature during handling of the mixed powder and liquid. Monomer can vaporize during heat-curing of the resin if the temperature rises too much. Curing under pressure helps keep the monomer from evaporating during polymerization and creates a denser acrylic. When chemical-cured acrylic is cured in room temperature water and 15 to 20 pounds of air pressure in a pressure pot (Figure 17-2), the resulting acrylic is stronger and has less porosity and shrinkage. A pressure pot is a laboratory device that resembles a pressure cooker. It is a thick-sided metal pot that seals well and can be pressurized by the addition of air through a one-way valve in its lid. Porosity can also occur by the entrapment of air during mixing of the powder and liquid acrylic resin components.
Allergic Reaction
Sensitive patients can react to the components of the denture materials. If excessive free monomer is present in the denture base, some people’s tissues may be irritated and inflamed by the methacrylate monomer. Other components such as hydroquinone, benzoyl peroxide, and pigments can also cause irritation. Dental personnel who work with the materials can develop contact dermatitis through repeated exposure of unprotected skin to the materials. Personal protective equipment (PPE) should be used when handling these materials. Dental resins are not known to cause systemic toxic reactions.
Acrylic Resin for Denture Bases
The functions of the acrylic resin denture base are to
• Retain the artificial teeth in the prosthesis
• Adapt to the supporting structures for stability
• Distribute forces of mastication over a wide area to reduce pressure on the ridges that might contribute to resorption of the underlying bone
• Replace missing tissues or rebuild the contours of tissues lost when the underlying bone resorbs
• Establish a seal along the periphery of a complete denture that aids in retention (Figure 17-3)
Polymerization Reaction
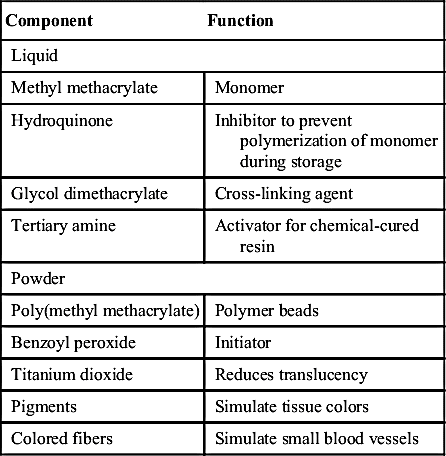
Acrylic resins polymerize by an addition reaction. Acrylic resins are supplied as a powder and a liquid. The powder is composed mostly of small beads of PMMA and benzoyl peroxide (the initiator). Inorganic pigments are added to give the acrylic resin colors resembling those of the oral mucosa, and titanium dioxide is added to keep the acrylic from being too transparent. Small, colored fibers may be added to simulate small blood vessels. Several shades of acrylic are available, so that the clinician can attempt to match the variations of racial pigmentation that can occur in the gingiva and mucosa of patients (Figure 17-4). The liquid contains MMA, hydroquinone as an inhibitor or preservative to prevent polymerization of the MMA during storage, and glycol dimethacrylate as a cross-linking agent. Cross-linking of the acrylic polymer chains helps prevent surface cracks, and it improves resistance to structural fatigue that can lead to fracture. The liquid is supplied in dark brown bottles to prevent ultraviolet light from initiating polymerization during storage. When the powder and the liquid are mixed, chemical- and heat-cured materials go through a similar reaction, except that chemical-cured materials have a tertiary amine in the liquid as an activator, whereas heat-cured materials do not (Table 17-2).
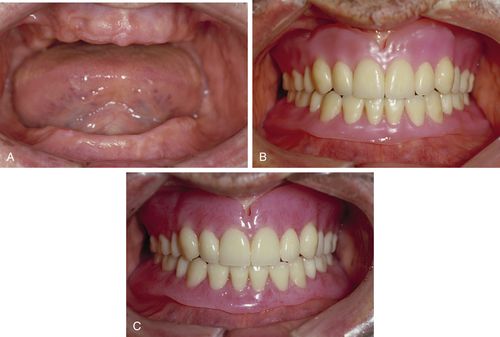
FIGURE 17-3 Complete dentures. A, Edentulous ridges. B, Denture teeth set in wax for try-in. C, Dentures processed in acrylic, polished, and delivered to patient.

FIGURE 17-4 Acrylic shade guides for matching the color of the gingiva.
(Courtesy of Mark Dellinges, School of Dentistry, University of California, San Francisco [San Francisco, CA].)
Physical Stages of Polymerization
Sandy Stage
The first stage seen when the powder (polymer) and the liquid (monomer) are mixed is called the sandy stage, because the mixture looks grainy, similar to sand and water, and has a runny consistency.
Stringy Stage
The second stage occurs when the powder particles absorb the liquid into their surface. It is called the stringy stage, because the mixture is stringy when handled and is thicker in consistency.
Dough Stage
In the next stage, called the dough stage, more of the powder goes into solution and the mixture changes from stringy to doughy and is more easily manipulated.
Rubber Stage
In the final stage, called the rubber stage, the mixture has a rubbery consistency that can no longer be manipulated for forming the denture base.
Chemical-Cured Resins
Polymerization of chemical-cured acrylic resins is set into action when the tertiary amine in the liquid activates the benzoyl peroxide in the powder to produce free radicals. The hydroquinone initially inhibits the reaction from progressing by destroying free radicals. This inhibition increases the working time so that the materials can be manipulated for a reasonable period of time, usually while the material progresses from the stringy stage to the dough stage. As the hydroquinone is depleted, the reaction proceeds more rapidly and advances from the dough stage to the rubber stage. The reaction is exothermic (releases heat) and ultimately becomes quite hot. When the reaction is complete, the material is hard and stiff.
Pour Technique
Some chemical-cured materials can be mixed to a thin, fluid consistency and poured into a mold and cured under pressure.
Heat-Cured Resins
The most common method for processing denture bases uses heat and pressure during polymerization of the acrylic resin. Heat-cured acrylic resins go through initial stages of polymerization that are similar to those of chemical-cured resins. After the dentist has tried in the denture teeth set in wax and the patient approves the appearance, the denture is returned to the laboratory, where the technician places the cast of the edentulous arch, along with the denture setup (teeth mounted in wax on a record base), in a specially designed processing flask. The flask separates in the middle into two sections. The denture setup mounted on the cast is invested in a plaster investing material in the flask in such a manner that the flask can be opened in the middle to allow removal of the wax and record base after heating in a water bath. The teeth are held in place by the plaster. All remnants of the wax are removed. A liquid, called a separating medium, is placed and air-dried on the plaster and the cast to prevent the acrylic resin from sticking to or absorbing moisture from the gypsum materials. Mixed acrylic resin in the dough stage is placed in the space created by removal of the wax and record base. (It has a longer dough stage than chemical-cured acrylic, because it does not have the tertiary amine activator.) This space is where the denture base will be formed. A sheet of polyethylene material is placed over the resin and the flask is reassembled and closed under pressure. The flask is reopened to remove excess material that exudes out of the sides of the flask. This process is repeated until no excess material appears and the polyethylene sheet is removed. The flask is put into a device called a pneumatic press that maintains pressure on it, and it is placed into a temperature-controlled water bath for at least 8 hours. Heat activates the benzoyl peroxide, causing the formation of free radicals and allowing polymerization to occur. Applying pressure and controlling the heat minimize porosity by preventing the monomer from vaporizing. More of the monomer is consumed during the polymerization, so less free monomer is present in the cured denture base with heat-processed dentures. Similar to the chemical-cured resins, the material is hard and stiff when the polymerization is complete. It also shrinks when it is polymerized. Shrinkage is seen most readily in the palatal area and can cause the acrylic to lift from the cast as much as 0.25 mm. If a room temperature processing technique is used, shrinkage is less, but the properties of the denture are poorer initially, because of the presence of much more free monomer. The properties improve as the free monomer leaches out.
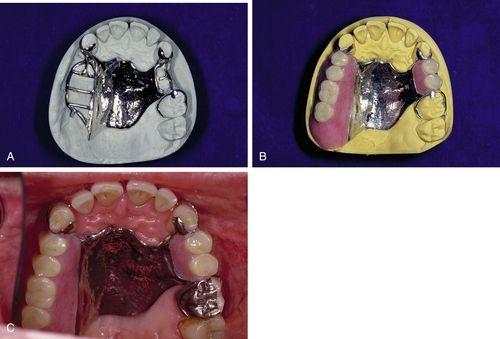
A similar process is performed for applying a denture base to a removable partial denture, except that the partial denture will have a metal framework invested in the mold, as well as the teeth set in wax. Because the acrylic resin does not adhere well to the metal, a retentive mesh or lattice is made as part of the partial denture framework to lock the acrylic in place (Figure 17-5).
Microwave Processing
The same denture resins used for the heat processing technique can be used for processing in a microwave oven. If a special monomer liquid is used in place of regular monomer, less porosity is found. Processing time is greatly speeded up, and processing is completed in about 5 minutes instead of the several hours needed for heat processing.
Injection Molding
Some vinyl acrylic resins can be processed by injecting the material into a mold when it is in a doughy form. With some materials, shrinkage and porosity are reduced by injection molding. Some laboratories use this technique because it is faster than the traditional heat processing.
Light-Cured Resins
In addition to heat- and chemical-curing, dental resins can be light-cured. The light-polymerized resins have photoinitiators such as camphorquinone and amine activators. These react to form free radicals when exposed to blue light and initiate the polymerization reaction (see Chapter 6). One of the commercially available light-cured materials (Triad VLC; Dentsply International) contains urethane dimethacrylate resin and silica fillers for thickening the material and reinforcing it. It comes in flat sheets or ropes depending on the application. Each sheet is individually packaged in a thick black plastic bag to prevent room light from reaching it and causing premature polymerization. The light-cured materials are fast and easy to use but require the purchase of an expensive light-curing unit (see Figure 17-23 in Procedure 17-2). The material is available in limited acrylic colors. It is used for denture bases, record bases (see Procedure 17-2), custom trays, and denture repairs. It also has applications for removable orthodontic appliances.
Denture Reline Materials (Liners)
Relining a denture is done for several reasons. A reline will fix looseness that occurs as the bony ridges resorb and the gums shrink. An upper denture will occasionally crack lengthwise through the palatal area as the ridges resorb. The reline readapts the denture to the ridges and repairs the fracture. A soft temporary reline material may act as a tissue conditioner to improve the health of the tissues before a hard reline is done. Some soft temporary reline materials will flow gradually to adapt to the tissues when worn for a day or two and will serve as a functional impression for a hard reline. A reline may serve to prolong the life of the denture as an alternative to making a new one.
Long-term denture relines can be made with either a hard setting material or a soft, flexible one. Both types of materials can be applied to the denture at chairside or may be sent to the dental laboratory for processing. In general, the laboratory processed reline gives a better result and will last longer. The chairside relines are more porous, more likely to break down sooner, and will stain more readily. The laboratory processed reline requires that the patient leave the denture out for 12 to 24 hours to allow the tissues to return to an uncompressed state before taking the impression for the reline: Pressure from wearing and eating with the denture will compress the tissues.
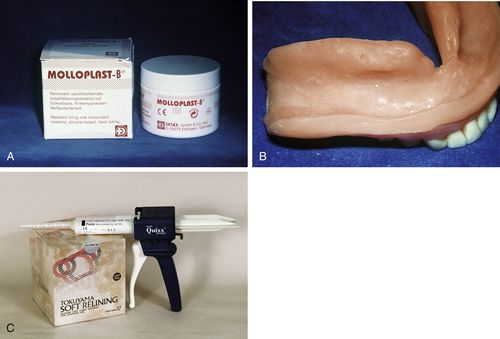
FIGURE 17-6 Long-term soft liners.
A, Heat-cured, silicone-based soft lining material. B, Soft liner processed in lower denture. C, Chairside, chemical-cured, silicone-based soft liner in cartridges dispensed with a mixing gun and tip. (Courtesy of Mark Dellinges, School of Dentistry, University of California, San Francisco [San Francisco, CA].)
Soft Relining Materials
Complete and partial dentures sometimes have a soft lining material placed on the tissue-bearing surface of the denture base. Soft liners may be used as short-term treatment for a few days up to a few weeks to allow tissues to heal after surgery, or when denture sores have formed. Soft liners may also be used for long-term treatment for patients who have chronic soreness with hard denture bases because of sharp bony spicules or thin mucosa over the ridges. Patients feel more comfortable with a lining that has a cushioning effect, especially if the denture is opposing natural teeth. Long-term soft liners are also used for patients with bony tissue undercuts that cannot be surgically corrected. A hard denture base rubs the mucosa in the area of the undercut, whereas a soft liner cushions the tissues and will flex in and out of the undercut when the denture is placed and removed. Soft liners are used with palatal defects such as cleft palate or tissue loss from cancer surgery or trauma. On average, long-term soft liner materials last about 1 to 3 years.
Long-Term Soft Liners
Long-term soft liners are made from silicone rubber or acrylics such as ethyl or methyl methacrylate that have been made pliable by the addition of plasticizers such as aromatic esters and alcohol (Figure 17-6). Long-term liners may be processed at room temperature or with the application of heat. Although some long-term silicone and acrylic liners can be placed at chairside, others are placed at the commercial laboratory. Heat-cured silicone liners are processed in the laboratory, because they release acetic acid that can cause tissue burns. Laboratory-processed relines are denser and last longer (1 to 3 years). Chairside soft relines are more porous and stain more easily. Long-term liners composed of acrylic will harden over time as the plasticizers leach out and will need replacement. The silicone liners are more stable over the long term, because they do not have softeners to leach out. However, they can be difficult to adjust. Special burs and stones are needed to make these adjustments. Another problem with long-term soft liners is that they often do not form a good bond to old acrylic. Therefore, they may separate from the denture base at the edges and leak between the liner and the denture base.
Silicone liners support the growth of yeasts such as Candida albicans and may cause tissue irritation that requires antifungal therapy. Cleaning soft liners on a daily basis in benzalkonium chloride will reduce the growth of yeasts.
Short-Term Soft Liners (Tissue Conditioners)
Short-term soft liners are referred to as tissue conditioners or treatment liners and are usually placed at chairside. They are supplied as a powder composed of poly(ethyl methacrylate) and softeners or plasticizers of aromatic oils and ethanol (Figure 17-7).
Application of short-term liner
The powder and liquid are mixed thoroughly according to the manufacturer’s directions and are flowed onto the tissue-bearing surface of the denture, which was previously cleaned with soap and water. The denture is reseated in the patient’s mouth, and the patient is instructed to gently close into normal occlusion until the material cures (see sequence in Figure 17-8). These liners are capable of readapting to the patient’s tissues as they heal, because they have a high degree of flow. Because this flow property is greatest the first day, hard foods should be avoided to prevent distortion of the material. As the plasticizers leach out, the resin becomes stiffer. The plasticizers leach out more quickly in the short-term liners than in the long-term liners and therefore need frequent replacement. Some short-term liners last for only 1 week; others last for 2 to 4 weeks.
Use of appropriate denture soaks can prolong the useful life of soft liners, and therefore the manufacturer’s recommendations are important. The dental assistant and hygienist must be familiar with materials and procedures for the placement of liners at chairside and for the home care of both chairside and laboratory-processed liners.
Hard Relining Materials
Immediate dentures are those placed immediately after extraction of the teeth. They become loose rapidly (usually within 6 to 12 months) as the extraction sites heal and the bone resorbs. These loose dentures can often be made to fit well again by placement of a hard liner
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses