JOINT PHYSIOLOGY AND PATHOPHYSIOLOGY
Motion is a fundamental event throughout biology. It is an especially powerful modulator of cellular events altering and interfering with the effects of cytokines and growth factors in the musculoskeletal system. Until recently, mechanical effects on joint tissue, especially the activation of cells, such as chondrocytes, synovial fibroblasts, and osteoblasts, were considered beneficial only in disease-free tissue and regarded as detrimental in the presence of inflammatory disease. When assessing the literature on the temporomandibular joint (TMJ), usually in place of motion, the term mechanical stress is used in association with the breakdown of joint function.
This provides a small glimpse of results and work of the last 10 years regarding the interaction of motion and inflammation in articular tissue in vitro and in vivo. It reveals how motion provides antiinflammatory effects even in inflamed articular tissue through intracellular mechanisms. It further outlines how motion-based therapies, such as continuous passive motion (CPM), exert beneficial effects in different states of inflamed joint tissues. The in vitro model compares chondrocyte behavior when chondrocytes are subjected to four different conditions: (1) inflammation (interleukin-1 [IL-1]), (2) motion (cyclic tensile strain [CTS]), (3) inflammation and motion, and (4) experimental control. The in vivo model compares inflamed cartilage behavior when cartilage is subjected to two different conditions: (1) immobilization (IMM) and (2) motion (CPM).
Understanding this can help in the clinical decision-making progress regarding therapeutic regimens of medical, surgical, and/or physical therapy interventions used in the treatment of temporomandibular disorders (TMD). It can also help in understanding the molecular basis of joint diseases.
INTERPLAY OF CYTOKINES, GROWTH FACTORS, AND MECHANICAL SIGNALS
Articular cartilage maintains normal TMJ function through a specialized, hydrated extracellular matrix (ECM). The major constituents of fibrocartilage include type II collagen fibrils providing tensile strength and aggregating proteoglycans (aggrecans), nonaggregating proteoglycans, and water providing compressive resilience. Less abundant components, types IX and XI collagen and small proteoglycans, also influence matrix organization.
Glycosaminoglycans are the polysaccharide side chains attached to the core proteins of proteoglycans. The distribution of proteoglycans close to the inferior and superior surfaces in a given articular tissue is not uniform. Chondroitin sulfate is evenly distributed in cartilage; dermatan sulfate is largely concentrated in the periphery and keratan sulfate in the middle of the TMJ disks. This may reflect different loading patterns in various regions of the TMJ disk.
Chondrocytes embedded in the articular cartilage express distinct functional properties in development and aging while maintaining homeostasis in the mature organism and during cartilage remodeling after traumatic or inflammatory injury. Physiologically, these responses lead to the formation or restitution of cartilage ECM. Dysregulation of these responses can lead to qualitatively or quantitatively abnormal formation or degradation of ECM, resulting in impaired joint function.
Additionally, mechanical stimuli affect articular chondrocyte metabolism. During normal daily activity, a variety of mechanical forces, including stresses, strains, and pressures, is distributed within the joint, and the long-term stability of the cartilage matrix depends mainly on the response of chondrocytes to mechanical stimuli and their limited capability to balance between anabolic and catabolic processes.
Chondrocyte activation and the induction of anabolic or catabolic responses are thought to be primarily the function of cytokines and growth factors. The catabolic cascade is induced by proinflammatory stimuli, such as IL-1 and tumor necrosis factor alpha (TNF α), and characterized by the secretion of proteases, suppression of matrix synthesis, and inhibition of chondrocyte proliferation. The anabolic process is associated with the secretion of antagonistic cytokines, synthesis of protease inhibitors, production of ECM, and cell replication.
During inflammation in degenerative TMJ diseases, proinflammatory cytokines, such as IL-1 and TNF-α, decrease synthesis and increase degradation of proteoglycans and collagens. Growth factors, such as transforming growth factor-beta (TGF-β), are unable to stimulate chondrocyte synthesis of collagens and proteoglycans and ultimately reduce the activity of IL-l–stimulated proteinases.
It is most important to note that mechanical signals generated by a tensile strain of low magnitude (transcutaneous electrical nerve stimulation [TENS]-L) are antiinflammatory, whereas a tensile strain of high magnitude (TENS-H) is proinflammatory and catabolic. Both low and high magnitudes of mechanical strain exploit nuclear factor (NF)- K B as a common pathway for transcriptional inhibition or activation of proinflammatory genes and catabolic processes as described for the intracellular mechanism of action of applied tensile forces in osteoblast-like cells. TENS-L is a potent inhibitor of IL-1β-induced I-?Bβ degradation and prevents dissociation of NF- K B from cytoplasmic complexes and thus its nuclear translocation. This leads to sustained suppression of IL-1β-induced NF- K B transcriptional regulation of proinflammatory genes. In contrast, TENS-H is a proinflammatory signal that induces I-?Bβ degradation, nuclear translocation of NF- K B, and transcriptional activation of proinflammatory genes ( Figure 45-1 ).
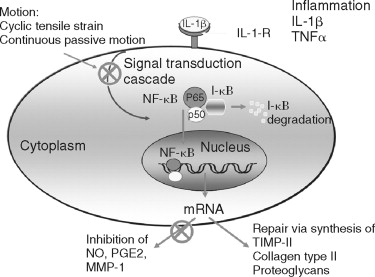
Articular chondrocytes synthesize copious amounts of the free radical nitrous oxide (NO) following activation by IL-l. NO is generated by the NO synthase (NOS) group of enzymes, which synthesize NO by combining molecular oxygen with the terminal guanidino nitrogen of the amino acid L-arginine, yielding L-citrulline as a co-product. Under aerobic conditions, NO spontaneously oxidizes to its stable metabolites, nitrate (NO 3 ) and nitrite (NO 2 ). Since the half-life of NO is on the order of seconds, NO production is assessed indirectly by measuring the conversion of 4 C-labeled L-arginine to L-citrulline in tissue homogenates or by measuring the accumulation of the stable NO metabolites, NO 3 and NO 2 , in biologic fluids, such as the synovial fluid. To date, three distinct isoforms of NOS have been identified: a neuronal form (nNOS) initially isolated from brain, an endothelial form (ecNOS) initially isolated from the endothelium, and an inducible form (iNOS) initially isolated from macrophages.
The iNOS pathway is principally regulated at the transcriptional level. Proinflammatory cytokines and endotoxin are potent inducers of iNOS in a wide variety of cell types, whereas glucocorticoids and the antiinflammatory cytokines, interleukin-4 (IL-4), interleukin-10 (IL-10), and the growth factor TGF-β suppress NO production. NO, acting as an endogenous mediator of catabolic actions, inhibits proteoglycan synthesis directly, whereas TGF-β suppresses that NO production reversing the NO-mediated inhibition of proteoglycan synthesis.
The synovial fluid of arthritic TMJs also contains inflammatory mediators, such as IL-1β, TNF, NO, leukotriene B 4 , and prostaglandin E 2 . Elevated levels of IL-1 and NO in the pathogenesis of arthritic conditions signal downstream events including the induction of matrix metalloproteinases (MMPs), eventually leading to the destruction of stromal tissue.
MMPs represent a family of structurally and functionally related enzymes responsible for the proteolytic degradation of ECM. They have been traditionally subdivided into three classes based on their substrate specificity: collagenases, gelatinases, and stromelysins. Their substrates include fibrillar collagen; collagen type IV, or denature collagen (gelatin); and other ECMs (proteoglycan, laminin, fibronectin, casein), respectively. MMPs have been detected in TMJ synovial fluid aspirates and lavage collections confirming that MMPs are involved in the degenerative component of TMJ disorders.
To treat acutely and chronically inflamed joints both IMM and mobilization are used as therapeutic measures with variable success. This is partly due to the fact that mechanisms by which these two differing treatments exert their effects remain unclear. Recent in vitro studies demonstrate that biomechanical signals are potent antiinflammatory signals that attenuate proinflammatory gene induction in chondrocytes. These signals act on chondrocytes in a magnitude-dependent manner. At low magnitudes, biomechanical signals inhibit IL-1β- or TNF α-induced transcriptional activation of cyclooxygenase-2 (COX-2), MMPs, IL-1β, and other proinflammatory molecules. These mediators are involved in cartilage destruction, and by blocking their gene expression, biomechanical signals can potentially attenuate cartilage destruction. Additionally, biomechanical signals up-regulate the expression of proteins associated with cartilage repair, such as aggrecans, collagen type II, and sulfated glycosaminoglycans. Not surprisingly, mechanical signals intercept the IL-Iβ–signaling pathway and inhibit nuclear translocation of NF- K B and subsequent transcriptional activation of genes under its control. Because NF- K B transactivation occurs within a few minutes following activation of chondrocytes with IL-1β or TNF-α, the actions of biomechanical signals are rapid and can be observed within a few hours. Moreover, biomechanical signals act on cells in a sustained manner and can be observed in the presence of an inflammatory stimulus. Additionally, mechanistic analysis of the actions of biomechanical signals in vivo is indicated to understand the biologic basis of motion-based therapies that may contribute significantly to the healing of inflamed joints.
THE ARTHRITIC CONDITION AND CYCLIC TENSILE STRAIN IN VITRO
CTS Versus CTS + IL-1 Versus Experimental Control Group Versus IL-1 Alone
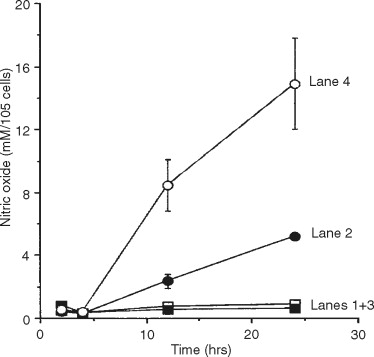
Mimicking the arthritic condition in vitro, chondrocyte function has to be assessed under at least four different conditions: (1) motion (CTS), (2) motion and inflammation (IL-1, IL-1), (3) inflammation (IL-1) alone, and (4) experimental control ( Figure 45-2 ). Exposure of chondrocytes to the proinflammatory cytokine IL-1 leads to the up-regulation of the presence of iNOS mRNA and iNOS protein and NO itself via the activation of IL-1 receptors on the cell surface and following dissociation of NF- K B from cytoplasmic complexes and thus its nuclear translocation ( lane 4 ). In particular, Figure 45-2 depicts that CTS itself does not induce the inflammatory cascade for NO ( lane 1 ), whereas CTS is clearly down-regulating the inflammatory pathway of NO ( lane 2 ). Resting chondrocytes in the control group do not show activation of the proinflammatory NO signaling cascade ( lane 3 ).
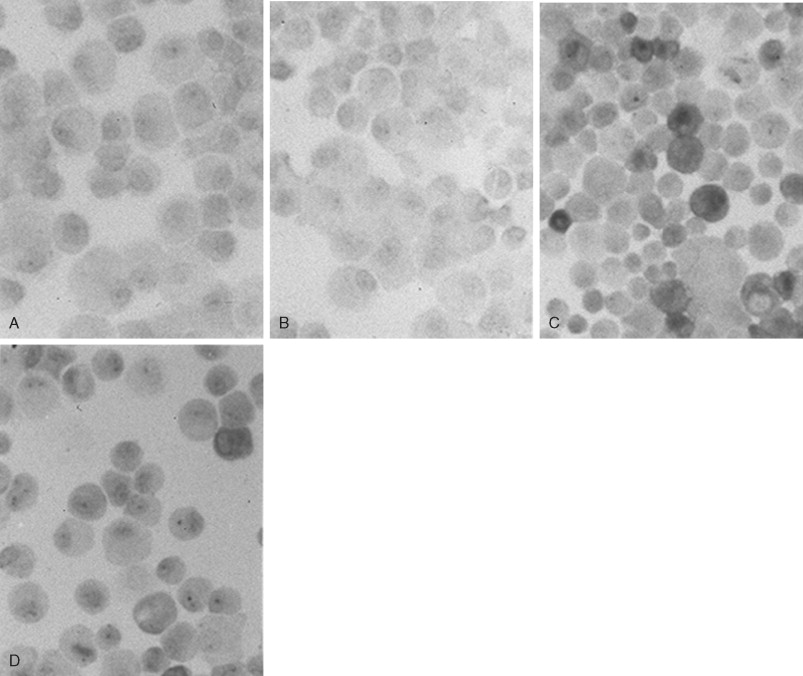
Chondrocytes in the tangential layers of cartilage are most responsive to IL-1β and synthesize high levels of iNOS as compared with chondrocytes in the deeper layers. As shown in Figure 45-3 , immunochemical staining for iNOS shows the distinct presence of an inflammatory response in chondrocytes following exposure to inflammatory signals alone in comparison with simultaneous exposure to mechanical and inflammatory signals. Absence of the iNOS protein occurs in control groups and during CTS alone ( Figure 45-3, A, B ). About 35% of all chondrocytes are iNOS-positive cells representing the cells derived from the tangential layer of articular cartilage ( Figure 45-3, C ). This population of chondrocytes is most likely the one that is also responsive to CTS showing a 62% decrease of iNOS protein immunostaining ( Figure 45-3, D ).
These in vitro findings provide evidence that mechanical signals play an important and critical role as modulators of inflammatory joint diseases. CTS functions as a potent effector mechanism for suppressing the pathologic effects of IL-1β in vitro through inhibition of inducible NO production. It is likely that in vivo mechanical signals may exert their effects on arthritic joints in a similar manner by inhibiting the inflammatory effects induced by IL-1β via interruption of iNOS expression. In turn, this would limit the production of NO and its catabolic effects on cartilage. Furthermore, mechanical signals do not only inhibit NO production, but also up-regulate proteoglycan synthesis at concentrations of IL-Iβ frequently observed in inflamed arthritic joints, suggesting that the actions of mechanical signals are clinically relevant in suppressing the sustained effects of pathologic levels of IL-1β in vivo. Maintaining anabolic capabilities of chondrocytes within inflamed joint tissue is an additional antiinflammatory asset for the treatment of arthritic conditions.
THE ARTHRITIC CONDITION AND CONTINUOUS PASSIVE MOTION IN VIVO
Immobilization Versus Continuous Passive Motion
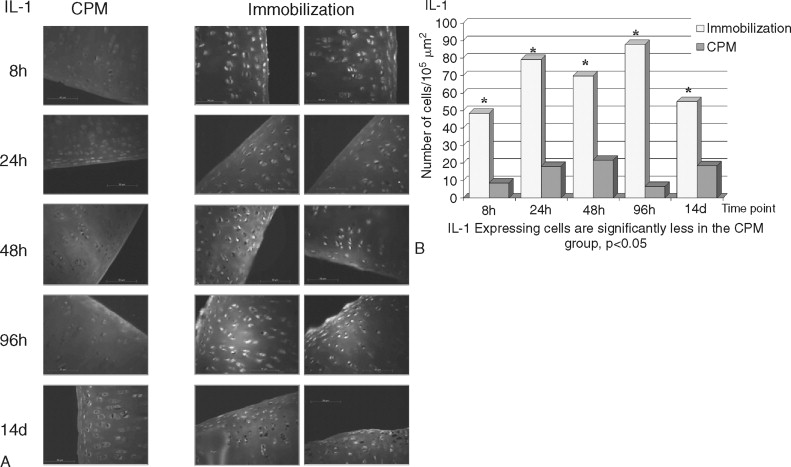
The destruction of cartilage integrity is a hallmark of arthritic conditions as evidenced in a rabbit model of antigen-induced arthritis (AIA). The histopathogenesis of AIA has been well characterized, and the model demonstrates many features of human rheumatoid arthritis (RA), including joint inflammation and damage to articular cartilage via both increased breakdown and decreased synthesis of cartilaginous matrix. To evaluate the molecular basis of interaction between motion and inflammation, the in vivo model compares inflamed cartilage behavior where cartilage is subjected to either of two different conditions: (1) IMM or (2) motion (CPM). Given that maintenance of cartilage integrity is due to chondrocyte function, regulation, and control of proinflammatory molecules in inflamed cartilage depends on the presence of biomechanical signals. Mobilization clearly prevents cartilage degradation. CPM protects matrix integrity in AIA-afflicted articular cartilage. Inflammation is mainly driven by monocytic cells in immobilized knees as compared with CPM knees. CPM down-regulates the proinflammatory cytokine IL-1β mRNA expression and synthesis in inflamed chondrocytes ( Figure 45-4 ). CPM suppresses COX-2 synthesis in inflamed cartilage (data not shown). Finally, CPM suppresses MMP-1 synthesis in inflamed chondrocytes ( Figure 45-5 ). CPM up-regulates IL-10 synthesis in inflamed cartilage ( Figure 45-6 ).
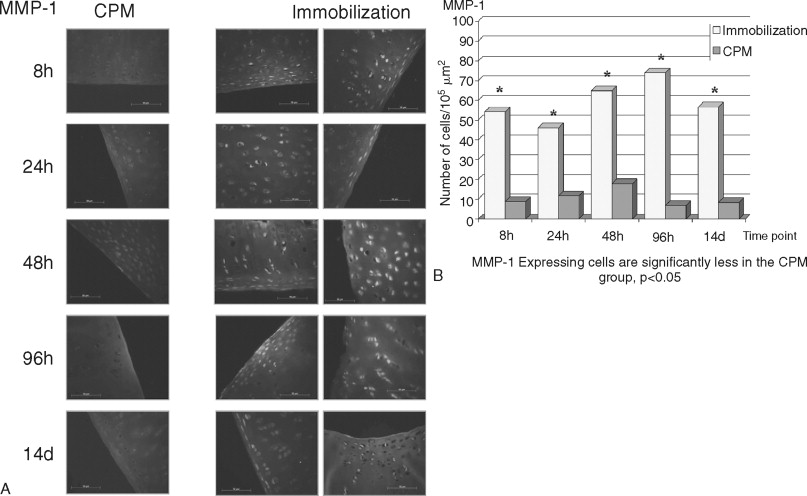
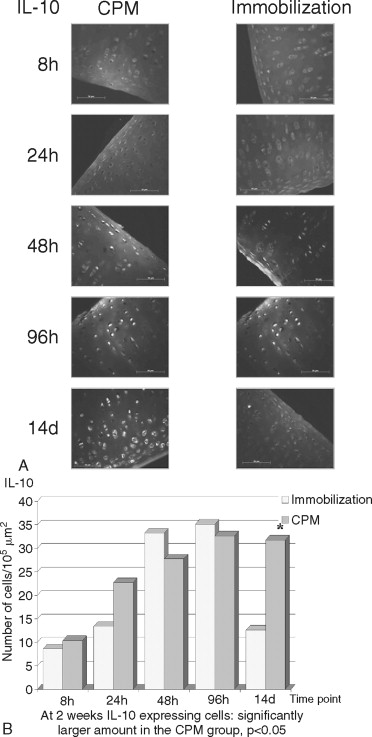
Joint mobilization is suggested to disseminate the inflammatory exudates, whereas IMM is suggested to create a stagnant environment in the synovium, leading to increased inflammation and cartilage damage. Although these effects of mobilization and IMM cannot be excluded, the fact that mechanical signals directly interfere with the proinflammatory gene induction in chondrocytes suggests that mechanical signals regulate inflammation at the intracellular level. The in vitro and ex vivo data demonstrate that chondrocytes are mechanosensitive cells and respond to mechanical signals in a magnitude-dependent manner. For example, cyclic tensile or compressive forces in the presence of inflammatory signals induce matrix synthesis in chondrocytes and fibrochondrocytes. The in vivo findings confirm the in vitro and ex vivo data and demonstrate that mechanical signals act directly on chondrocytes to prevent cartilage destruction caused by inflammation. Appropriate mechanical signals play a profound role in attenuating localized joint inflammation in vivo. These signals act on inflamed chondrocytes by two equally important mechanisms. First, these signals are potent inhibitors of proinflammatory gene induction and inhibit the expression of catabolic mediators (e.g., IL-1β, COX-2, and MMP-l). Second, these signals induce the expression of the antiinflammatory cytokine IL-l0. Together, the results demonstrate that biomechanical signals have profound effects in minimizing cartilage destruction in a severe proinflammatory environment and provide continuing evidence that motion-based therapies are more efficacious than perceived earlier.
ARTHRITIDES OF THE TMJ
More than 10% of the population suffers from arthritic diseases leading to pain, loss of function, disabilities, and joint replacement and economically measured as a major loss of human productivity and especially early retirement. Alone in the United States, more than 40 million people suffer from some form of arthritis; among these, 12 million have osteoarthritis (OA) and 8 million have RA. Arthritis is also the most common disease affecting the TMJ. All of the arthritic forms that involve other joints in the body also occur in this joint. Therefore these conditions should be considered in the differential diagnosis whenever a patient has chronic TMJ pain and dysfunction.
Arthritic diseases of the TMJ are inflammation-derived alterations of the synovium, cartilage, and surrounding structures, such as the capsule, bursa, tendons, and bone. Similarities of the signs and symptoms of the many variants of arthritis necessitate the establishment of a working differential diagnosis based on the patient’s history, physical examination, and radiographic evaluation delineating these conditions from other causes of TMD symptoms.
Patient evaluation includes questions regarding swelling, redness, pain at rest, duration of symptoms, rate of onset, location of involved joints, pattern of joint involvement, preceding events such as trauma, and time of day when symptoms are worst. A discussion of medications influencing symptoms and a thorough review of organ systems follows. Physical examination extends beyond the involved joints because the skin, heart, lungs, and mucous membranes are often affected by the same disease process. Involved joints are observed for deformity, symmetry, and proximity. Palpation of these areas assesses pain, temperature, and the range of motion.
Diagnostic studies also include radiologic studies (magnetic resonance imaging [MRI], computed tomography [CT] scan, and bone scans), laboratory tests, and synovial fluid analysis.
Normal synovial fluid is clear or straw colored and viscous from hyaluronic acid. Viscosity is reduced in inflammatory arthritides, and the synovial fluid flows like water—unlike the stringing produced by normal synovial fluid. The evaluation of cell counts, clot formation, and differences between serum and synovial glucose levels can aid in the diagnosis and differentiate arthritic conditions. The presence of crystals and the evaluation of their size, shape, and compensation under polarized light can be useful in the diagnosis of metabolic arthritides.
CLASSIFICATION OF ARTHRITIS
The symptoms and signs of the different arthritic diseases are similar, yet clinically individual conditions can be distinguished.
- 1.
Traumatic arthritis
- 2.
Degenerative (osteo)arthritis
- 3.
Inflammatory arthritis
- a.
RA
- b.
Juvenile rheumatoid arthritis (JRA)
- c.
Psoriatic arthritis (PA)
- d.
Ankylosing spondylitis (AS)
- e.
Reiter syndrome (RS)
- a.
- 4.
Infectious arthritis
- a.
Gonococcal arthritis
- b.
Syphilitic arthritis
- c.
Tuberculous arthritis
- d.
Lyme disease–associated arthritis
- a.
- 5.
Metabolic arthritis
- a.
Gout
- b.
Pseudogout
- a.
- 6.
Arthritides associated with connective tissue diseases: systemic lupus erythematosus (SLE), scleroderma, chronic inflammatory bowel disease, reflex sympathetic dystrophy, sarcoidosis
TRAUMATIC ARTHRITIS
The immediate intraarticular response of the joint tissues to single or repetitive episodes of acute trauma is the release of proinflammatory cytokines starting a cascade of inflammatory events. This response, traumatic arthritis, is manifested in the form of pathologic and clinical states, the nature of which is generally determined by the magnitude of the trauma sustained by the joint tissues. Minor trauma to the joint tissue may result in compression or shearing of the retrodiskal tissue and capsule or dislocation of the disk.
Moderate trauma may cause damage to the synovial lining, resulting in subsequent edema and mild serohemorrhagic effusion that can cause hemarthrosis and an inflammatory response in the synovial membrane. This inflammation may eventually result in a hypertrophic synovial response and fibrosis, causing cicatricial contractions and adhesions between the joint components and even fibrous ankylosis. In children, traumatic arthritis can cause growth abnormalities, malocclusion, and facial asymmetry. Severe trauma , short of producing a condylar fracture, can cause damage to the articular surfaces, subchondral bone, or both, eventually predisposing to or causing degenerative joint disease.
Clinical Findings
Acute traumatic arthritis presents with severe jaw pain both at rest and with movement. The joint is tender to palpation, occasionally swollen, and has a decreased range of motion. Effusion in the joint space and edema or hemorrhage in the retrodiskal tissue can cause an increased distance between the articulating components on the traumatized side that results in a posterior open bite. The acute symptoms usually subside within a few days or weeks. In cases of severe trauma, however, the condition may eventually progress to persistent limitation of motion, ganglion formation, intraarticular disk derangement, or degenerative joint disease.
Imaging Findings
As a result of edema and swelling, the intracapsular and capsular structures can develop an increase in thickness. This can be reflected radiographically as an increased distance between the roof of the glenoid fossa and the condylar surface in the closed-mouth position. Late severe traumatic changes in the joint may be manifested as degenerative joint disease or as bony ankylosis.
Diagnosis
A thorough history must be taken and a careful physical examination of the TMJ must be performed after injury to exclude the presence of condylar or other mandibular fractures. The diagnosis of traumatic arthritis of the TMJ is based on a history of trauma and on the absence of the characteristic features of the other forms of arthritis. Trauma to the TMJ can aggravate the inflammatory status of other preexisting forms of arthritis. The use of arthroscopy to evaluate the intraarticular disk and to lavage the joint after condylar trauma has been reported, but the long-term benefit of such a procedure is still uncertain.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


