Fig. 13.1
Influence of different bisphosphonates on angiogenesis in a murine Matrigel angiogenesis model: (a) control group, (b) treatment with clodronate, (c) ibandronate, (d) pamidronate, and (e) zoledronate. Especially nitrogen-containing bisphosphonates reduce angiogenesis
On the other hand, angiogenesis during bone formation was not significantly altered by bisphosphonates [2, 21, 22]. However, vascularity might well play a role in the pathogenesis of the disease and especially with regard to the frequency of occurrence in the mandible and the maxilla [23], but it is not likely to be the key factor in the pathogenesis because it cannot be explained why only the jawbones are affected – especially when having in mind that maxilla and mandible have a completely different pattern of vascularity – while there are only few cases reported in other bones of the human body [2, 24–26].
Furthermore, it has been discussed that after local accumulation of bisphosphonates and combined with other cancer medications, bisphosphonates might exert direct soft tissue toxicity towards the oral mucosa. This could lead to mucosal injury and jawbone exposure as seen in MRONJ [2, 27–29]. Also soft tissue toxicity might play a role in the pathogenesis of MRONJ, and mucosal healing is delayed after dento-alveolar surgeries in patients receiving bisphosphonates, but jawbone exposure is not constantly present in all cases of BRONJ/MRONJ proven by histology especially not in early stages (stage 0 according to AAOMS 2009 and 2014), and some radiological and clinical symptoms like pain and impairment of the inferior alveolar nerve function can occur when mucosal integrity is still intact [1, 2, 30, 31].
While all of these theories might play a role in the pathogenesis of BRONJ/MRONJ, none of them neither in isolation nor in combination are able to provide satisfactory answers for questions of paramount importance:
Why is the jawbone almost exclusively the target for BRONJ/MRONJ?
Why are nitrogen-containing bisphosphonates associated with a much higher risk for the development of BRONJ/MRONJ when compared to non-nitrogen-containing bisphosphonates?
What is the role of the proposed risk factors and the so-called “trigger events” in the development of BRONJ/MRONJ? [1, 2, 23].
Effects of Local Inflammations and pH on BRONJ/MRONJ Pathogenesis
In order to be able to answer the above-mentioned questions, one has to deal with the special features of bisphosphonates on the one hand and of the jawbone on the other hand. Bisphosphonates have the special property of selective uptake by their target organ, namely, the bone. While bisphosphonates bind to the hydroxyapatite at neutral pH values, they are released and activated in acidic milieus [2]. This well-known mechanism takes place physiologically in Howship’s lacunae when the bone is resorbed by osteoclasts when the dissociation between the bone (hydroxyapatite) and bisphosphonates is increased in acidic pH values [1, 2, 23, 32]. Up until now, this known mechanism has not been brought into connection with the pathophysiology of bisphosphonate-related osteonecrosis of the jaws although it might play a substantial role in the multi-factorial aetiology of BRONJ/MRONJ. Supporting this, it was already shown in 1991 by Sato and colleagues that alendronate is released in acidic milieus in a rat model [2, 33]. Clinically, acidic milieus commonly occur in the course of infections and during wound healing after surgical procedures [34, 35]. Such conditions occur more often in the jawbones due to the frequency of marginal and apical infections and dento-alveolar surgeries, especially tooth extractions. Thus, these infections can lead to the acidification of localised jawbone areas, resulting in the release and activation especially of nitrogen-containing bisphosphonates into potentially toxic levels which can finally lead to BRONJ/MRONJ (see Fig. 13.2) [1, 2, 32, 36]. This cascade of processes might as well occur after pressure sores and micro-traumata or even “spontaneously” depending on the duration of intake and route of administration (cumulative dose present in the bone) combined with other potential risk factors such as co-morbidities and co-medications. Non-nitrogen-containing bisphosphonates (e.g. clodronate and etidronate) having lower antiresorptive potencies in general are not activated by these processes. This is in line with the fact that despite decades of clinical use, there are only few reported cases of BRONJ due to the intake of non-nitrogen-containing bisphosphonates [1, 2, 37].
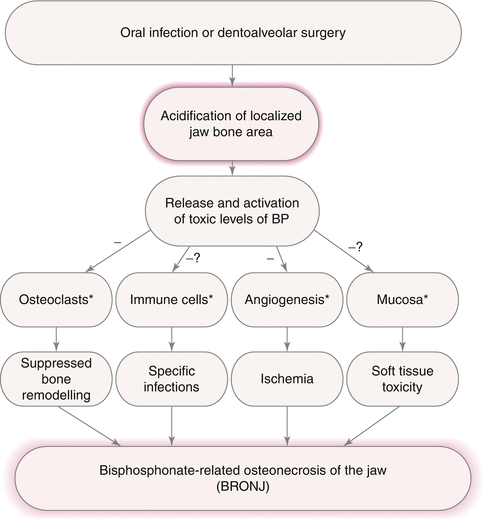

Fig. 13.2
Schematic diagram of the potential pathogenesis of bisphosphonate-related osteonecrosis of jaw (BRONJ) with the pH-value reduction as a crucial activator. The minus signs symbolise inhibition of the following processes or tissues; the question marks identify the cursorily investigated pathogenesis theories. The asterisks depict the points where risk factors (smoking, diabetes, steroids, chemotherapy, poor oral hygiene, co-morbidity) might aggravate the BRONJ pathogenesis [2] (Reprinted from Otto et al. [2] with kind permission of Elsevier)
Once a critical concentration of bisphosphonates in solution is reached, there is not only an inhibitory effect on the target cells of bisphosphonate treatment, namely, osteoclasts, but there is an inhibitory and potentially even toxic effect on a number of other cell types including mesenchymal stem cells, osteoblasts and osteocytes as well as endothelial cells, mucosal cells and immuno-competent cells including macrophages and T-lymphocytes [38, 39]. At this point, all the above-mentioned theories regarding BRONJ/MRONJ development come into play (Fig. 13.2). So, in fact, other existing theories can be linked with this theory [1, 2].
Not only does the above-described theory explain why the jawbone is an almost exclusive target of BRONJ/MRONJ and why apical and marginal infections as well as dento-alveolar surgeries and especially nitrogen-containing bisphosphonates can trigger the occurrence of BRONJ/MRONJ. But it also offers a thorough rationalisation of why chemotherapy, immuno-suppression and systemic disorders such as diabetes can increase the risk for BRONJ/MRONJ. The reason is that these circumstances are associated with a higher risk of wound healing disturbances and local infections [2, 40–42].
Experimental Data Supporting the Role of Local Inflammations and pH Changes in the Pathogenesis of BRONJ
Indeed, cell cultural data could prove that increasing doses of nitrogen-containing bisphosphonates in solution lead to severe reduction in cell survival and activity of human mesenchymal stem cells while equimolar concentrations of the non-nitrogen-containing bisphosphonate clodronate did not show significant inhibitory effects (see Fig. 13.3) [1]. This is in line with the clinical finding that the majority of BRONJ/MRONJ cases occurred after long-term intravenous treatment with zoledronic acid while there are hardly any cases of ONJ under treatment with non-nitrogen-containing bisphosphonates even though they have been in clinical use for decades [23, 37].
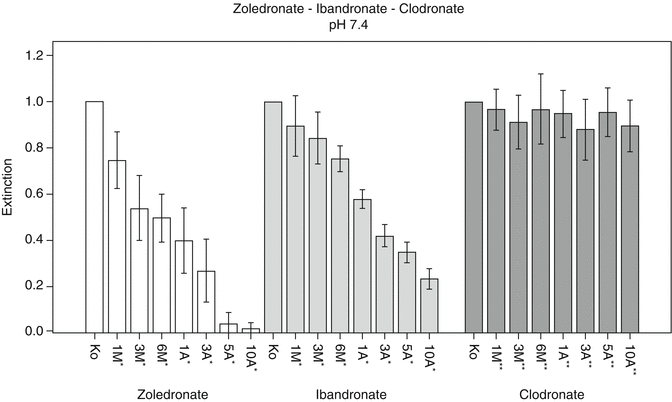

Fig. 13.3
Quantitative analysis of cell viability and cell activity using WST assay at pH 7.4 for the two N-BPs, zoledronate and ibandronate, and the non-N-BP, clodronate. Zoledronate: control vs. 6-month exposure equivalent p < 0.01, control vs. 3-year exposure equivalent p < 0.01 and 6 month- vs. 3-year exposure equivalent p < 0.01. Ibandronate: control vs. 6-month exposure equivalent p < 0.01, control vs. 3-year exposure equivalent p < 0.01 and 6-month vs. 3-year exposure equivalent p < 0.01. Clodronate: control vs. 6-month exposure equivalent p = 0.214, control vs. 3-year exposure equivalent p = 0.05 and 6-month vs. 3-year exposure equivalent p = 0.038. *Dose-adjusted concentrations equivalent to a treatment in standard oncology dose. **Equimolar concentrations of clodronate as calculated for dose-adjusted concentrations of zoledronate [1] (Reprinted from Otto et al. [1] with kind permission of Elsevier)
Moreover, these inhibitory effects on cell survival, activity and motility were more pronounced for nitrogen-containing bisphosphonates when pH decreased to 6.2 – comparable to local inflammations in the human body (see Fig. 13.4) [1]. This is in line with the clinical finding that the majority of BRONJ/MRONJ cases are preceded by inflammatory conditions [43].
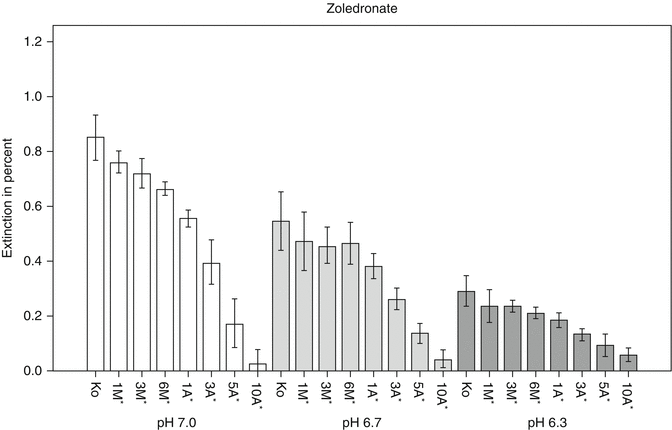

Fig. 13.4
Quantitative analysis of cell viability and activity using WST assay at pH 7.0, 6.7 and 6.3 for zoledronate. Values are expressed as percentage of activity at pH 7.4, which was set to 1.0 in all cases. *Dose-adjusted concentrations equivalent to a treatment in standard oncology dose. **Equimolar concentrations of clodronate as calculated for dose-adjusted concentrations of zoledronate [1] (Reprinted from Otto et al. [1] with kind permission of Elsevier)
Furthermore, the group of Agis could prove that once bisphosphonates are bound to the hydroxyapatite, they are completely inert while the same concentration of nitrogen-containing bisphosphonates in solution showed toxic effects towards fibroblasts [44]. The physiological mechanism which brings bisphosphonates which are normally bound to the hydroxyapatite of the bone in solution is local acidification which occurs during inflammatory processes. This condition has a crucial role for the vitality of the jawbone. In addition to inflammatory odontogenic infections, the oral microenvironment becomes acidic with very frequent intake of various foods and drinks [45]. The buffering and washing capacity of saliva plays an important role in the maintenance of the pH level of the oral flora. Although the oral cavity has a naturally low alkaline pH level, it is often influenced by decreases in pH levels in other parts of the body. The buffering capacity of saliva might be insufficient to protect the oral flora-bone-soft tissue junction microenvironment in cases of excessive decreases at pH level which leads to BP release from the bone and probable infection with necrosis in related structures that could explain why BRONJ/MRONJ affects mainly the jawbones [46].
In fact, a recent animal study performed on rats could prove that an alkaline environment achieved using local applications of sodium bicarbonate has a preventive effect with regard to the development of BRONJ/MRONJ after tooth extractions. The authors concluded as well that local inflammations might play a key role for the pathogenesis of BRONJ [46]. Furthermore, Aguirre and co-workers could prove that ONJ like lesions developed in areas with pre-existing periodontitis after the administration of oncological doses of zoledronic acid in rats without tooth extractions or other surgical interventions [47]. Recently, a large animal model for BRONJ in mini pigs has been developed, and studies regarding the effect of local infections in large animal models are on the way [48]. Considering the results obtained from these studies, the presence of a damaged gap (either traumatically or periodontally) between the bone and oral flora which causes infection may found to be inductive for the onset of the disease.
Clinical Data Supporting the Role of Local Inflammations and pH Changes in the Pathogenesis of BRONJ
Besides that there are numerous clinical proofs that local infections play a crucial role for the pathogenesis of medication-related osteonecrosis of the jaws, Dimopoulos et al. showed that implementation of preventive measures prior to the start of bisphosphonate treatment in multiple myeloma patients could significantly reduce the risk of BRONJ [49]. Riplamonti and colleagues could confirm this significant decrease of BRONJ/MRONJ occurrence if preventive measures aiming in avoidance of local infections were implemented prior to the start of bisphosphonate treatment in patients with solid tumours. Even if prophylactic measurements were implemented after the start of bisphosphonate treatment, the risk for BRONJ was lower and lesions could be detected earlier [50].
Besides that, all expert panels and studies recommend to perform dento-alveolar surgeries in patients receiving bisphosphonates in a way that avoids local wound infections in order to prevent the occurrence of BRONJ/MRONJ. If dento-alveolar surgeries in bisphosphonate patients are performed under antibiotic prophylaxis and using local mucosal or mucoperiosteal flaps, the occurrence of BRONJ/MRONJ is significantly reduced.
Furthermore, the progression of the disease can be stopped or at least reduced in the majority of cases simply by local (mouth rinse) and systemic (antibiotics) disinfective measurements, and the treatment of MRONJ cases is basically aimed at the removal of necrotic and infected bone parts accompanied by local mucoperiosteal or flap closure and antibiotic treatment. All of these clinical data give a strong hint for the role of inflammation and infection in the occurrence and progression of the disease.
Besides, there some general considerations that seem to prove the role of pH value with regard to the effects as well as the side effects of bisphosphonates. When taken orally, the main side effect occurs in the gastro-intestinal tract because bisphosphonates are in solution in the strong acidic milieu of the stomach due to gastric acid. When given intravenously, the main side effect occurs in the kidney where once again bisphosphonates are in solution and where there is an acidic milieu of the primary urine close to the tubulus cells.
The increasing numbers of recent publications dealing with the occurrence of osteonecrotic lesions of the external ear channel, a location which is also prone to infections, and the report of occurrence in bone transplants in the jawbone area [51] are more arguments for the crucial role of the local milieu and infections for the development of MRONJ rather than the jawbone or jawbone turnover alone.
Osteonecrosis Due To RANKL-Inhibitor and VEGF-Inhibitor Treatment
In the last years, new anti-resorptive substances for patients with bone metastases and osteoporotic patients have been used clinically. One class is RANK-ligand inhibitors, namely, denosumab. In normal condition, osteoblasts can activate osteoclast differentiation and clonal expansion via the secretion of RANK ligand. This balance can be disturbed in pathophysiological conditions. Tumour cells can activate osteoblasts via cytokine secretion, increasing RANK-ligand production. This results in an increased bone resorption by osteoclasts, which plays a key role in the development of bone metastasis. RANK-ligand inhibitors disable this pathophysiologic mechanism. Some anti-angiogenic drugs were also used in cancer patients, alone or in combination with bisphosphonates, and are under the cloud to trigger osteonecrosis of the jaw. The real incidence of medication-related osteonecrosis of the jaw besides bisphosphonate-related osteonecrosis of the jaw is still unknown. Several case series of osteonecrosis of the jaw in patients with cancer who underwent denosumab therapy have been reported in the recent literature, and seemingly, the overall incidence of denosumab-related osteonecrosis of the jaw is similar to that for nitrogen-containing bisphosphonates in this population [52
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


