Reference
Year
Type of study
Cause of BP treatment
No. of BRONJ patients
Control group
BP type in BRONJ
Risk factors significantly associated with BRONJ development
Sedghizadeh et al. [83]
2013
Retrospective study (population pharmacokinetic model)
Osteoporosis and cancer
69
84
A, 55 %
Longer duration BP, older age, and Asian race
Z, 20 % (Oral 71 %)
Thumbigere-Math et al. [32]
2012
Retrospective study
Carcinoma (MM, breast and prostate carcinoma)
18
558
Z, 55.5 %
High risk in zoledronate, hypothyroidism, smoking, diabetes, longer duration, and increased number of BP treatments
P + Z, 39 %
Vahtsevanos et al. [44]
2009
Longitudinal cohort (with vs without ONJ)
MM and cancer
80
1,541
Z, 97.5 %
Higher risk in dental extraction, denture use. More patients with MM than breast cancer, ibandronate and pamidronate showed a lower risk than zoledronate
P, 20.0 %
I, 3.8 %
Fehm et al. [50]
2009
Retrospective study
Breast carcinoma
10
335
Z, 70 %
Increased number and duration of BP
Mauri et al. [120]
2009
Meta-analysis from 15 RCT
Adjuvant breast carcinoma Tx.
13
5312
Z, 100 %
High risk in zoledronate
Boonyapakorn et al. [41]
2008
Prospective study
MM & carcinoma
22
58
Z, 64 %;
High risk in zoledronate (3.5 times higher than others), MM &and breast cancer, long-term use (>2.5 years) increases risk
P, 14 %
Wessel et al. [66]
2008
Case–control
Carcinoma
30
150
High risk in zoledronate, obesity, and smoking
Jadu et al. [45]
2007
Retrospective study (only P treatment)
MM
24
120
P, 100 %
Longer duration of BP, dental extractions, cyclophosphamide therapy, prednisone therapy, erythropoietin therapy, low hemoglobin levels, renal dialysis, and advanced age
Dimopoulos et al. [42]
2006
Prospective study
MM
15
187
Z, 47 %
High risk in zoledronate
P + Z, 40 %
Bamias et al. [29]
2005
Retrospective study
Carcinoma (MM, breast, and prostate carcinoma)
17
252
Z, 41 %
Total number and duration of BP exposure, high risk in zoledronate
P + Z, 53 %
Bisphosphonate Administration Itself: Type, Drug, Route, and Dose
The chemical structure of amino-bisphosphonates is characterized by the existence of nitrogen in the R-side chain, which ensures a stronger potency of the drug [3]. There is no doubt that these nitrogen-containing bisphosphonates predominantly cause BRONJ compared with non-nitrogen-containing bisphosphonates. Therefore, most of the studies on BRONJ focus on nitrogen-containing bisphosphonates (zoledronate, pamidronate, ibandronate, alendronate, risedronate, etc.) [27]. It has been reported that intravenous bisphosphonate administration (e.g., zoledronate and pamidronate) carries a higher risk of ONJ development compared with oral bisphosphonates [19, 28–32]. Bisphosphonates are actively prescribed to patients diagnosed with metastatic breast cancer, prostate cancer or multiple myeloma (MM), and the related hypercalcemia [33]. To inhibit osteolytic activity and treat the hypercalcemia from metastatic bone disease, bisphosphonates are widely used to prevent pathological fracture and pain from malignant bone disease. According to a review of previously published case series of 368 BRONJ patients (1966–2006), zoledronic acid comprised 35 % and pamidronate comprised 31 % of the total cases. Oral bisphosphonates (alendronate, risedronate, ibandronate) comprised only 4.8 % of the total cases [34]. The lower incidence of BRONJ by oral bisphosphonates could be attributed to differences in pharmacological efficiency between oral and intravenous bisphosphonates. Oral bisphosphonates have a low absorption rate (<1 %) in the gastrointestinal tract, whereas more than 50 % of the intravenous bisphosphonates are incorporated into the bone [35, 36]. The dose of bisphosphonates for cancer treatment is up to 12 times higher than for osteoporosis [37, 38].
Intravenous Bisphosphonates
Many reports have revealed zoledronate to be associated with a high risk of osteonecrosis compared with pamidronate or other bisphosphonates [39–43]. Vahtsevanos et al. [44] and Hoff et al. [43] reported that having a history of zoledronic acid treatment increased 15-fold the relative risk of BRONJ. Pamidronate-related BRONJ data have shown that each additional year of administration increased the BRONJ risk up to 1.7 times [45]. This has been attributed to the more potent inhibitory action of zoledronate on bone turnover than that of pamidronate [46–48]. Zoledronate has an inhibitory effect on the bone turnover rate that is 10–100 times more powerful [46]. However, BP potency itself cannot explain all the reasons for the higher risk of BRONJ development after zoledronate treatment compared with pamidronate. For example, the relative inhibition of bone remodeling for ibandronate is ten times more than that of pamidronate [49]. However, ibandronate showed a 92 % reduced risk of BRONJ development in a longitudinal cohort study [44] or a very low incidence of BRONJ development when it was administered as a single medication [48]. Thumbigere-Math et al. [32] also reported that there was no significant difference in the cumulative dose of ibandronate in patients treated with ibandronate, whereas zoledronate and pamidronate had a significantly higher mean cumulative dose.
Among the patients exposed to intravenous bisphosphonates, around 1 % of the patients developed BRONJ at 1 year after treatment, which increased to 13 % at 4 years cumulatively [42]. Another report also showed that the cumulative danger of developing BRONJ reached 1 % after 1 year of administration and up to 20 % after 3 years [41]. Prolonged duration of BP administration [29, 32, 42, 43, 50] and an increased cumulative dose have been reported to be significant risk factors for BRONJ [32, 43, 48, 50], especially for longer durations of zoledronate treatment [44]. The median exposure time for BRONJ development was 12–24 months for zoledronate, 19–30 months for pamidronate, and 13–21.5 months for ibandronate [32, 41–43]. It is unknown why BRONJ is more frequently reported in multiple myeloma and breast cancer patients than in prostate and renal cancer patients and in patients with Paget’s disease. A possible explanation is the relatively longer duration and greater cumulative dose of bisphosphonate medication for multiple myeloma and breast cancer patients than that for the other diseases [43]. In general, development of BRONJ is associated with the combined effect of dose, duration, and potency of the bisphosphonates [43].
Oral Bisphosphonates
The reported incidence of orally induced BRONJ is very low. According to the data from the Merck company, the estimated incidence of BRONJ after alendronate treatment was 0.7 cases per 100,000 person-years’ exposure [51]. A population-based study from Australia [30] showed that weekly alendronate can possibly result in 0.01–0.04 % of cases developing BRONJ. A recent epidemiological investigation by a mail survey revealed a 0.1 % prevalence of BRONJ after oral bisphosphonate intake [52], which is higher than previously reported data from the company. Case–control studies have shown that oral treatment with BP is the definitive risk factor for ONJ [53, 54]. ONJ related to oral bisphosphonates comprised 15.3 % of the total number of BRONJ cases reported (a review of the literature 2003–2009 by Filleul et al. [55]) and was 7.8 % of the cases in an European multicenter study [56] and 39.5 % of the cases in a Japanese multicenter study [57]. Most of the BRONJ cases derived from oral BP treatment (oral BRONJ) were related to alendronate [52, 58–61].
Because the prevalence of oral BRONJ is quite low, it is difficult to determine significant risk factors. According to the American Association of Oral and Maxillofacial Surgeons (AAOMS) position paper [2], oral BRONJ risk increases when the duration of intake exceeds 3 years. However, there was no supporting scientific evidence for this notion. A nationwide survey in Japan [57] showed that the duration of bisphosphonate administration before ONJ onset was 33.2 months (0.2–135 months), whereas it was 23.6 months (1.2–103 months) for intravenous bisphosphonate intake. Fleisher et al. [62] reported a median 3 years for intravenous bisphosphonates and 5 years for oral bisphosphonates before the onset of ONJ. Other reports also showed similar periods of time before the onset of oral BRONJ: mean 48.8 months [53], 57.8 months [56], 66.5 months [60] or median 52.8 months [61]. Barasch et al. [54] reported that BRONJ risk begins within 2 years of bisphosphonate administration for both cancer and osteoporosis patients. This risk of BRONJ in non-cancer patients increased substantially after 5 years of BP treatment. These data reveal that oral BP-related ONJ was usually reported to develop between 2.5 and 5.5 years after BP treatment. This implies that oral BRONJ can also exist even without a long incubation time. Therefore, patients receiving oral BP for more than 2.5–3 years should be closely monitored.
Bisphosphonates have a long half-life. In particular, alendronate suppresses the bone turnover marker up to 5 years after cessation of the drug [63]. Patients without malignant bone disease receive BP for longer periods of time and may have a greater possibility of accumulating a higher dose of BP. If these BP-treated patients experience local risk factors, the number of BRONJ cases could be increased in the future.
Systemic Risk Factors (Underlying Disease, Co-Morbidities, and Co-Medications)
Intravenous Bisphosphonate Intake
Initial reports for case series of BRONJ showed that the medical comorbidities of the patients were chemotherapy, corticosteroid use, diabetes, smoking, alcohol abuse, low body weight, menopause, and old age [19]. Usually, chemotherapeutic agents and corticosteroids are commonly used for metastatic bone disease, with delayed wound healing being one of the inevitable adverse side effects of these treatments [24]. According to Jadu et al. [45], antineoplastic drugs (cyclophosphamide) and corticosteroids increased the risk of BRONJ in multiple myeloma patients. In some reports, chemotherapy was significantly related to BRONJ development [32, 41, 48]. However, high-dose chemotherapy accompanies long-term BP treatment, and it is difficult to define delayed wound healing or bone exposure that would be attributed solely to the chemotherapy. Corticosteroid treatment has been reported to be one of the significant risk factors for BRONJ in a retrospective case–control study [45]. However, other reports have failed to find a statistical difference between the number of steroid users in BRONJ and the control group [29, 41, 43, 64–66]. The dose and duration of the corticosteroid treatment varies widely depending on the patients’ disease and condition; thus, the contribution of corticosteroids to BRONJ development needs further investigation with a proper study design.
Other systemic risk factors are erythropoietin therapy [45], renal dialysis [45], and diabetes [32, 67]. Hypothyroidism was also reported to be associated with BRONJ development [32]. The exact mechanism of such risk factors in BRONJ onset needs to be investigated further. However, asthma, dyslipidemia, and hypertension have not been reported to be significant risk factors [36, 67].
Oral Bisphosphonate Intake
It has been proven that oral BP increases ONJ risk [53, 54, 68]. However, in some reports, there was no significant effect of oral BP on ONJ development [66, 69]. This might be attributed to the limited number of cohort studies or case–control studies that can ensure an adequate level of scientific evidence. Therefore, the reported risk factor for BRONJ related to oral BP was based on the frequency of co-morbidity in a case series. Patients without systemic or local risk factors rarely develop BRONJ after oral bisphosphonate intake alone. A retrospective cohort study of 30 oral BRONJ patients [61] showed that diabetes (33 %) and systemic inflammatory disorders (20 %) involving long-term corticosteroid use (23 %) were the most common co-morbidities. A recent retrospective multicenter study showed that hypertension (40.2 %) and diabetes (9.2 %) were the major co-morbidities [58]. In some reports, hypertension or cardiac disease was the most frequent systemic disease in oral BRONJ patients [70, 71]. Another report revealed that patients with BRONJ from oral BP who had comorbid conditions experienced prolonged healing times and reduced healing [61]. Therefore, systemic comorbidities need to be considered before treatment. However, these are “systemic comorbidities” and more scientific supporting data should be accumulated to confirm the “significant risk factors” related to oral BRONJ.
Local Risk Factors (Infections, Extractions, Pressure Sores, etc.)
It has been clearly shown that dental risk factors such as invasive dental procedures (dental extraction), denture irritation, and periodontitis are related to BRONJ development [65]. Hoff et al. [43] reported that dental extraction increases the hazard ratio 9.9 times in multiple myeloma patients and 53.2 times in breast cancer patients. A longitudinal cohort study in cancer patients also showed an 18-fold elevated risk of BRONJ after extraction and a two-fold increase after denture irritation [44]. Dental surgical procedures increased the incidence of BRONJ as high as 5.3-fold [45] or seven-fold [64].
The healing process of a dental extraction site reflects the systemic wound healing capacity. Blood clot formation after extraction leads to granulated tissue and finally mineralization to an osseous structure. Because bisphosphonates accumulate in skeletal sites with high bone turnover, such as the maxilla or mandible [72], BP-bound osseous tissue resorbs slowly. This bacterially contaminated bone cannot be readily resorbed, and this prolonged open wound increases the risk of bacterial invasion. This can result in a favorable environment for the development of chronic osteomyelitis [73, 74].
Periodontal disease is one of the possible risk factors for BRONJ. Inflammation generally decreases the pH level and this acidic milieu leads to the protonated activation of nitrogen-containing BP [75, 76]. A recent investigation with a periodontitis-associated microbe showed that periodontitis is one of the significant risk factors for BRONJ in cancer patients [77].
The incidence of mandibular BRONJ is higher than that for maxillary BRONJ [19, 28, 41] and it is more common at sites with thin overlying mucosa, such as exostoses, the sharp mylohyoid ridge, and the mandibular torus [2, 78].
According to the literature, around 20 % [58], 28 % [59], 33 % [61], and 57 % [56] of oral BRONJ can be “spontaneous BRONJ,” which means the absence of systemic or local risk factors or co-morbidities in BRONJ development. Further studies are needed for this type of BRONJ. Some authors have reported that osteonecrosis does not begin with aseptic necrosis, but is in fact “osteomyelitis from the very beginning” [79]. However, the presence of this spontaneous BRONJ supports the theory, at least in part, that osteonecrosis is primarily an aseptic process and that an infection develops afterward [80, 81].
Host Factor
Age has been suggested to be one of the risk factors for BRONJ in malignant bone disease [29, 40, 45, 82]. Each additional year of life increases the risk by 1.1 times [45]. However, a report has shown that after statistical adjustment, old age did not increase the BRONJ risk in a cohort study [44]. Gender was not significantly associated with BRONJ [2]. Smoking has been proposed to be a significant risk factor [32, 66]. Obesity has also been suggested to be a risk factor [66]. Sedghizadeh et al. [83] carried out a pharmacokinetic study and showed that Asians are more susceptible to BRONJ than Caucasians, Hispanics, and African–Americans. The authors suggested that Asians have a lower body weight, and a smaller skeletal compartment that might result in drug accumulation and thereby, higher concentrations over time, as well as increased toxicity. However, there is no consensus on the reported risk factors related to these host factors.
Genetic Risk Factors
Various factors have been suggested to be related to BRONJ development. A possible association with genetic factors has also been proposed by several investigators. Several genome-wide association studies [84, 85] have proposed that the single nucleotide polymorphism (SNP) of the cytochrome P450, the subfamily of the 2C polypeptide 8 (CYP2C8, rs1934951), in multiple myeloma patients, showed a significant association with BRONJ. CYP2C8 is mainly expressed in the liver and known to be related to drug metabolism and clearance [86]. However, other researchers could not find such a relationship between BRONJ and CYP2C8 SNP [87, 88]. This inconsistency might be attributed to the fundamental limitation in collecting homogeneous case and control groups. Another genetic polymorphism in the RBMS3 (rs17024608) gene was suggested to carry a high risk of BRONJ development [89]. Because these genetic investigations need a large amount of genotyping to increase the statistical power, further large genetic studies are needed to identify susceptible genes involved in BRONJ development.
Surrogate Markers for BRONJ Risk
Bone remodeling is the combined process of bone resorption and bone formation. Prevention of skeletal-related events (SRE) of BP is attributed to reduced bone remodeling rather than bone formation [63]. Various bone turnover markers, such as CTX (C-terminal telopeptide), NTX (N-terminal telopeptide), PYD (pyridinoline), DPD (deoxypyridinoline), and P1NP (N-terminal propeptide of type 1 procollagen) are now widely used in clinical practice to diagnose specific bone diseases [90]. In particular, serum bone resorption markers have been utilized to determine the guidelines for osteoporosis treatment with BPs [91, 92]. BP treatment decreases bone turnover to 60–70 % below the baseline level [93].
It has been proposed that over-suppression of bone turnover may be related to the development of BRONJ [60] and bone turnover markers such as CTX have been recommended to determine the risk for BRONJ or to determine treatment options [60, 94]. Other reports also mentioned that CTX may be used for risk assessment [95]. However, these previous results were based on case series without a control group. Kunchur et al. [96] first reported the results of a case–control study. They reported that a CTX value of < 150 pg/ml did not correlate with the clinical risk factors of age, gender, co-morbidities, bone disease, or bisphosphonate duration, but stated that CTX can reflect a “risk zone (<150–200 pg/ml)” because the initial CTX values of BRONJ at the time of diagnosis were less than 200 pg/ml. If these are true, the CTX values can be used for assessing BRONJ risk and guidelines for a drug holiday, which may be beneficial in preventing BRONJ. However, various researchers greatly criticize the use of CTX as BRONJ marker, and their opinions have been expressed as ‘letters to the editor’ [97], case reports [98], or review articles [90, 99–101].
A recent case–control study with bisphosphonate patients reported that the serum CTX level has a limitation in showing BRONJ development [102, 103]. According to data from the author’s hospital, CTX level after long-term BP treatment in the control patients was not statistically different from the BRONJ patients (Fig. 3.1). Kim et al. [102] carried out a case–control study that compared 37 BRONJ patients with 37 age- and gender-matched control patients (BP cover > 24 months, but without ONJ). Along with osteocalcin, DPD, NTX, and bone-specific alkaline phosphatase, CTX value did not show any differences between the case and control groups. The result revealed that it is discouraged to use bone turnover markers for BRONJ risk estimation.
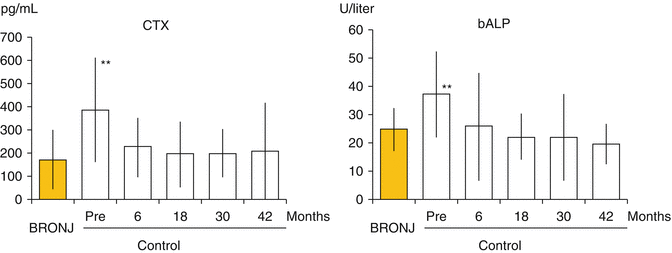

Fig 3.1
The bisphosphonates-related osteonecrosis of the jaw (BRONJ) group (n = 55, age 72.9 ± 7.3) comprised patients treated with oral bisphosphonates for postmenopausal osteoporosis who developed osteonecrosis of the jaw (ONJ) between January 2004 and December 2010 at the Department of Oral and Maxillofacial Surgery at Kyungpook National University Hospital. The control group (n = 85, age 71.6 ± 7.6) was treated with oral bisphosphonates (BPs) for osteoporosis at the Department of Orthopedics, but did not have any signs or symptoms of jaw necrosis. Biochemical markers of BRONJ patients at the time of diagnosis (yellow bar) compared with the control patients before (Pre) and 6, 18, 30, and 42 months after oral bisphosphonate (alendronate, risedronate, pamidronate, and ibandronate) administration (empty bars). CTX C-terminal telopeptide of collagen type I, bALP bone-specific alkaline phosphatase. (** p < 0.01, Comparison between BRONJ patients and individual time points of the control group (Reprinted with kind permission of Springer Science + Business Media))
Utilizing the surrogate markers of bone resorption to predict BRONJ risk has the following limitations. First, systemic bone turnover markers cannot readily reflect the maxillary and mandibular bone condition. Second, bone metastasis influences the CTX level in cancer patients. Therefore, the CTX level in these cancer patients covering BP is influenced by both factors; the BP administration itself and the bone metastasis condition [101, 104]. Third, according to histomorphometric analysis, some osteoporosis patients already show greatly suppressed bone remodeling before BP treatment [105]. Therefore, based on the current data, use of the CTX value as a predictor of BRONJ risk or disease progression cannot be supported.
In summary, nitrogen-containing bisphosphonates, intravenous route of administration, higher dose and longer duration of intake, and dental infections/dental surgical treatments can be regarded as “known” risk factors. However, other risk factors, particularly associated with oral BP-related ONJ, need further study with more sophisticated scientific investigation. Up to now, there has been no evidence for using bone resorption markers to predict BRONJ risk.
Risk Factors for Denosumab-Related ONJ
Denosumab is a human monoclonal antibody against the receptor activator of nuclear factor kappa-B ligand (RANKL). Denosumab inhibits the RANKL, an important mediator of osteoclastic differentiation [106]. As an antiresorptive agent, denosumab reduces osteoclastogenesis and is widely used for the treatment of metastatic bone disease and osteoporosis [107–110]. It had been proposed that inhibition of RANK–RANKL interaction by denosumab may also influence monocyte migration and decrease cell survival [111], which may be related to ONJ development. However, the exact similarities and difference between the BRONJ and denosumab-related ONJ (DRONJ) have not yet been clearly understood.
Denosumab is usually administered via subcutaneous injection of 60 mg every 6 months (for osteoporosis or prevention of skeletal-related events (SRE)) or 120 mg monthly (for oncological conditions or bone metastasis). Unlike bisphosphonates, denosumab is not incorporated into the bone matrix and has a relatively short half life. Even though the denosumab shows the higher efficacy in preventing skeletally related events and a lower rate of renal complications in cancer patients, the occurrence of the ONJ was similar (zoledronate 1.3 %, denosumab 1.8 %) [112] or rather higher but not statistically significantly higher (zoledronate 1 %, denosumab 2 %) [107] than in zoledronate-treated patients.
The reported incidence of DRONJ ranges from 0 % to 4.7 % [112–116]. In a recent meta-analysis of seven randomized controlled trials for 8,963 patients with solid tumors, such as prostate or breast cancer, the overall incidence of DRONJ was 1.7 % (95 % CI, 0.9–3.1 %). Denosumab administration increased the risk of ONJ development compared with a control placebo group (RR 16.28, 95 % CI: 1.68–158.05, p = 0.017), although the increase in risk between denosumab and bisphosphonates was not statistically significant (RR 1.48, 95 % CI: 0.96–2.29, p = 0.078) [117]. However, denosumab (60 mg) treatment every 6 months for prostate cancer patients did not result in any ONJ development (DRONJ, n = 0/1,468 patients) [115]. Another study also showed a very low incidence of ONJ (n = 2/2,207) after 60 mg of denosumab every 6 months for 2 years in patients with postmenopausal osteoporosis [118].
According to the analysis of 37 cases of BRONJ (zoledronate) and 52 cases of DRONJ, related oral events were tooth extraction (64.9 % in BRONJ, 59.6 % in DRONJ) and oral infection (45.9 % in BRONJ, 50.0 % in DRONJ). The mandible carried a higher risk than the maxilla (mandible:maxilla = 83.8 %:13.5 % in BRON, 65.4 %:28.8 % in DRONJ). The cumulative incidence of DRONJ was 0.8 % in the first year, 1.8 % in the second year, and 1.8 % in the third year, which was slightly higher but not statistically significantly higher than for BRONJ [116]. Denosumab for the treatment of giant cell tumors of the bone resulted in 1 % (n = 3/281) ONJ, which occurred roughly 13–20 months after treatment initiation [119].
Based on the limited number of studies in the current literature, the oncological dose of denosumab (monthly 120 mg), the intraoral surgical trauma, and the local site (mandible) may be suggested to be risk factors related to DRONJ. Therefore, the related risk factors for DRONJ may not be significantly different from those for BRONJ. Further investigation is needed to clarify the risk factors related to these antiresorptive drugs to minimize ONJ after drug administration.
In the process of malignant tumor development, angiogenesis is critical for tumor growth, infiltration, and distant/regional metastasis [121]. Recently, angiogenic inhibitors targeting the vascular endothelial growth factors (VEGF) with tyrosine kinase inhibitors (TKIs), monoclonal antibody or mammalian target of rapamycin (mTOR) pathway are used for chemotherapeutic agents to treat advanced carcinoma or metastatic bone disease [122]. Recombinant monoclonal immunoglobulin antibody, bevacizumab, blocks the all isoforms of VEGF-A and can suppress cancer progression and bone metastasis [123
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


