Fig. 14.1
Phases in wound healing. The wound healing process can be divided into three partly overlapping phases. Wound contraction is an early phenomenon, while scar formation is a late phenomenon in wound healing (arrows)
In intraoral wounds, the wound healing process is generally faster than in skin and generates less scar tissue. Therefore, intraoral wounds are sometimes considered to be more similar to fetal wounds (Okazaki et al. 2002). This may be related to the presence of lower levels of pro-inflammatory and pro-fibrotic cytokines in mucosal wounds (Szpaderska et al. 2003). The intraoral wound healing process is also influenced by the presence of saliva and large numbers of bacteria (Zelles et al. 1995). Saliva contains many growth factors such as epidermal growth factor (EGF). In addition, phenotypic differences between skin and mucosal fibroblasts may be involved (Lepekhin et al. 2002). These considerations, however, mainly apply to buccal mucosa, which has a quite different morphology than the palatal mucosa (Fig. 14.2).
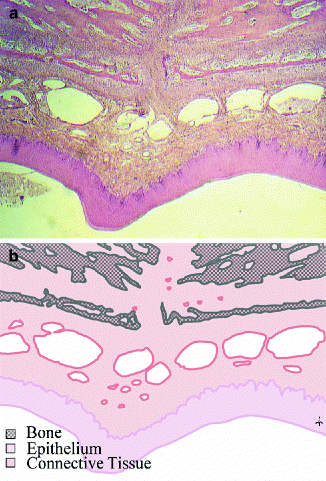

Fig. 14.2
(a, b) The palatal mucoperiosteum. The mucoperiosteum of the palate contains an epithelium and a submucosal connective tissue and is firmly attached to the palatal bone
In contrast to buccal mucosa, the palatal mucosa is a mucoperiosteum, which means that mucosa and periosteum are merged and attached to the palatal bone (Squier and Finkelstein 2003). The palatal mucoperiosteum is also much stiffer than buccal mucosa, and it contains less elastin fibers, as was also shown for the gingival mucoperiosteum (Bourke et al. 2000). Furthermore, the epithelium of palatal mucosa is generally thicker than in the buccal areas, and it is keratinized. All this implies that the physiological and mechanical characteristics of this tissue are quite different from buccal mucosa, which might explain differences in the outcome of the wound healing process.
Nevertheless, the general outline of both the palatal and the buccal wound healing process is similar to that of skin, which is described below (Clark 1996). It is important to stress that the phases of wound healing described here are not discrete episodes but they overlap in time (Fig. 14.1). In addition, the progress of healing in the outer wound area is more advanced than in the center, which means that subsequent phases of the wound healing process may be found in adjacent areas. This, of course, concerns only open wounds with a tissue defect in which healing takes place by second intention.
14.2.2 Phases in Wound Healing
Tissue injury causes disruption of blood vessels and bleeding. Within seconds, the coagulation cascade starts, leading to the formation of a fibrin-rich blood clot that contains numerous platelets. These platelets are reservoirs of cytokines and growth factors such as transforming growth factors (TGFs) and platelet-derived growth factor (PDGF) that attract inflammatory cells, especially neutrophils and macrophages. The blood clot forms a provisional matrix for the migration of those cells. Proteins such as fibronectin, fibrinogen, and vitronectin allow cell attachment and migration by their interaction with integrins, which are transmembrane cell surface receptors (Yamada and Clark 1996). Neutrophils and macrophages subsequently clear the wound bed of debris and bacteria. In addition, the relative blood volume at the site of injury increases by dilation of the capillary vessels in the surrounding tissue and also by an increase of their permeability, which leads to redness and swelling. This phase is called the inflammatory phase and usually subsides several days after wounding (Fig. 14.1).
In the next phase, the tissue formation phase, keratinocytes, fibroblasts, and endothelial cells in the wound edge start to proliferate. They migrate into the wound bed and start to form the neo-epithelium and the underlying granulation tissue (Clark 1996). This phase already starts a few days after wounding, before the inflammatory phase has come to an end. Keratinocytes seem to be activated by the partial loss of cell–cell contacts at the wound edge and by locally produced growth factors such as epidermal growth factors (EGFs) and fibroblast growth factors (FGFs). Fibroblasts, endothelial cells, and more macrophages migrate into the wound bed. Their migration and other activities are regulated by complex interactions with growth factors and extracellular matrix components within the provisional matrix (Yamada and Clark 1996). Again, integrins play a major role in the interaction between cells and the extracellular matrix. The binding of matrix proteins to integrins is required for attachment and migration, and it also leads to the transmission of additional regulatory signals into the cells (Rojas and Ahmed 1999). Cell migration furthermore requires the activity of matrix metalloproteinases (MMPs), enzymes that pave the way for cells by cleaving extracellular matrix proteins (McPherson 1992).
During migration into the wound, fibroblasts gradually switch to a more synthetic phenotype, a switch that involves the action of TGFβ. The fibroblasts start to produce large quantities of collagen, with collagen type III as the main species, but elastin is not synthesized in the wound. Within this matrix, endothelial cells form numerous capillaries to nourish the newly formed tissue. This process is called neo-angiogenesis and FGFs, vascular endothelial growth factor (VEGF), and many other growth factors are implicated in its regulation as shown by in vitro studies (Clark 1996). As soon as the wound is filled with granulation tissue and the neo-epidermis is formed, the collagen production is reduced, which requires interferon-γ (IFNγ). A negative feedback mechanism based on the accumulated collagen may also contribute to the decrease in collagen synthesis. This event marks the beginning of the third and last phase, the remodeling phase.
The remodeling phase starts within 1 week after wounding and will ultimately lead to the formation of scar tissue. The remodeling of the extracellular matrix is mainly carried out by fibroblasts. It involves the degradation of collagen type III by matrix metalloproteinases (Mignatti et al. 1996) and the concurrent deposition of type I collagen by fibroblasts. In the second week after injury, fibroblasts also start to produce proteoglycans. The mechanical properties of the tissue are not only determined by collagens but to a large extent also by these proteoglycans, since they can bind large amounts of water. In addition, many proteoglycans have been shown to regulate cell function, either by direct modulation of cell adhesion and proliferation or indirectly through the binding or release of growth factors (Nakato and Kimata 2002).
At the start of the remodeling phase, part of the fibroblasts within the granulation tissue differentiates into myofibroblasts that possess contractile properties. These specialized cells are strongly involved in the process of wound contraction. Their differentiation seems to be governed mainly by mechanical tension within the matrix, by TGFβ and by a specific variant of fibronectin, the ED-A fibronectin (Tomasek et al. 2002). Wound contraction causes a rapid reduction of the surface area of the wound and a concomitant rearrangement of the collagen fibers. In the mean time, the neo-epidermis is maturing into a fully differentiated stratified epithelium. After 1–2 weeks, no further contraction takes place because the myofibroblasts have disappeared, probably by apoptosis (Desmouliere et al. 1995). The induction of apoptosis is not completely understood, but several genes are known to govern the process. The expression of these genes is regulated by growth factors as well as by changes in the interaction between the cells and their extracellular matrix. In the next several months, many of the fibroblasts, but also endothelial cells, disappear by apoptosis, which gradually renders the tissue less vascularized and less cell rich. The slow remodeling of the collagen fibers by remaining fibroblasts, which is part of the transition to scar tissue, can go on for a long time.
14.2.3 Contraction and Scarring
Wound contraction and scarring seem to be the two main processes in wound healing responsible for the growth disturbances after cleft palate repair and are therefore reviewed here in more detail. Wound contraction is the reduction of the wound surface area by approximation of the wound edges, and it may account for up to 80–90 % of wound closure (McGrath and Simon 1983). The evolutionary function of this feature obviously is to speed up wound closure and thereby reduces the risk of infection and dehydration. Scar formation might be a negative side effect of this primarily beneficial process.
The cause of wound contraction is not yet exactly known. Two main theories have been described in literature. The first one states that fibroblasts, which migrate from the wound margins into the wound bed, cause traction in the extracellular matrix. This tensional force would be sufficient to contract the wound (Ehrlich and Rajaratnam 1990). This theory does not require specialized cells to explain wound contraction. The second theory assumes that a specialized subtype of fibroblasts, the myofibroblast, is responsible for wound contraction (Desmouliere and Gabbiani 1996; Gabbiani 2003). During wound contraction, fibroblasts, containing intracellular stress fibers are found within the granulation tissue. These stress fibers have been shown to contain alpha-smooth muscle actin (ASMA), a cytoskeletal protein also present in smooth muscle cells. This protein seems to be required for the contraction of myofibroblasts within the granulation tissue. The coordinated contraction of myofibroblasts, attached to the extracellular matrix, causes the reduction of the wound surface.
Nowadays, the two theories have merged into a consensus theory stating that both fibroblasts and myofibroblasts are involved in wound contraction (Tomasek et al. 2002). Initially, migrating fibroblasts in the wound area generate tension within the matrix. The resulting strain within the matrix triggers the differentiation of fibroblasts into myofibroblasts, which also requires the presence of TGFβ1. The coordinated action of myofibroblasts strongly increases tension within the wound tissue, which subsequently contracts. Thus, in the consensus theory, both fibroblasts and myofibroblasts contribute to wound contraction.
During contraction of the granulation tissue, extensive collagen remodeling takes place in which MMPs play a prominent role (Mignatti et al. 1996). As a consequence of this remodeling, collagen type III is gradually replaced by collagen type I. The new collagen is deposited in an orientation that is guided by the main lines of tension within the extracellular matrix (Huang et al. 1993; Rudolph et al. 1992). The reorientation of collagen fibers and the substitution of type I collagen for type III collagen mark the start of scar tissue formation. If a uniform direction of tension exists within the contracting granulation tissue, the new collagen fibers will also be deposited in a uniform orientation. Consequently, the resulting tissue will develop the properties of a scar, a process that may slowly progress for several months to years (Rudolph et al. 1992).
During scar tissue formation, the number of endothelial cells and fibroblasts within the developing scar tissue slowly decreases, a process in which apoptosis is involved. The final scar tissue therefore is poorly vascularized and has a low cell density. In addition, elastin fibers, which provide elasticity to normal mucosa and skin, are not resynthesized during wound healing and scar tissue formation. Their absence and the presence of highly oriented collagen fibers make the scar a rigid and stiff tissue. A specific feature of palatal wound healing is the attachment of the scar tissue to the palatal bone (see Sect. 14.4.1). This may cause palatal repair to have considerable effects on maxillary growth.
14.3 Effects of Palatal Repair on Growth
Apart from embryonic distortions and intrinsic growth deficiencies, facial growth in cleft lip and palate patients may be affected by surgical repair, orthodontic treatment, and functional adaptations (Kuijpers-Jagtman and Long 2000; Ross 1987a, b, c, d, e, f, g; Rygh and Tindlund 1982; Semb and Shaw 1996). Since the landmark studies of Graber (1949) and Dahl (1970), numerous descriptive cephalometric studies have been published (for an overview, see Semb and Shaw 1996). It is reasonably well established that cleft surgery, in particular lip and palate repair, can disturb normal growth and development of the maxilla in cleft patients (Berkowitz 1977; Kuijpers-Jagtman and Long 2000). However, the possible growth effects of surgery should be evaluated in relation to the intrinsic abnormalities of craniofacial growth in cleft palate patients. This requires that unoperated cleft lip and palate patients should be studied as well (Capelozza Filho et al. 1996; Derijcke et al. 1994; Lambrecht et al. 2000; Mars and Houston 1990).
Of all surgical procedures that are used in cleft lip and palate patients, palatal surgery has attracted the greatest amount of attention. The reason is that during this procedure, mucoperiosteal flaps are created on the palate to close the cleft, leaving areas of denuded bone. The scar tissue that is formed during healing might be a potential inhibitor of subsequent maxillary growth and dental arch development. Many studies over the past 50 years have focused on the effects of specific techniques of primary palate repair on midfacial growth and development. The effects of palatal closure seem to be mainly confined to the maxillary base and arch (Kuijpers-Jagtman and Long 2000; Semb and Shaw 1998). The maxilla is shown to be narrower, shorter, and displaced posteriorly relative to the cranial base. The dentoalveolar processes are often deflected to the median, resulting in anterior and transverse cross bites. However, since both lip and palatal surgery are generally performed, it is difficult to distinguish between the effects of the two types of surgery.
A problem with the evaluation of the literature on this subject is that the majority of the publications suffer from major methodological drawbacks, which minimizes their value (Ross 1987f; Kuijpers-Jagtman and Long 2000). Hardly, any studies are available that directly compare two types of treatment in a prospective research design. In contrast, there is a vast amount of retrospective studies available that all attempted to relate specific maxillary growth effects to particular surgical procedures. The most comprehensive study of this type is the multicenter study by Ross (1987a, b, c, d, e, f, g). By comparing lateral cephalometric radiographs collected from 15 cleft palate centers from around the world, they concluded that it was difficult to isolate the effects of individual palate repair techniques. However, an inhibition of anterior growth and translation of the maxilla was a common finding. Another problem that is only addressed by few is that not only the surgical technique but in particular the skills of the surgeon are very important for the long-term outcome in terms of growth and development (Kuijpers-Jagtman and Long 2000; Shaw et al. 2000).
In conclusion, strong consensus exists that primary surgery is a major factor in the impairment of dentomaxillary growth. The extent of growth impairment may be influenced by the specific techniques, the timing and sequence of operations, the use of orthopedic appliances, and possibly the most important of all, the skills of the surgeon. No particular technique has been shown to produce consistently better growth results than any other. Assuming that scar tissue is a primary etiological factor in maxillofacial growth disturbances, most contemporary repair techniques attempt to minimize scarring. Animal experiments are very well suited to determine the exact effects of specific surgical procedures. For a better understanding of the biological mechanisms in wound healing, and for the goal-directed modulation of healing, experimental studies are of major importance. Results of such studies are discussed in the next section.
14.4 Experimental Research
Extensive research in animal models has been performed to evaluate the effects of cleft surgery on growth and development of the maxilla and to study the wound healing process. Animal models are also used to develop new surgical techniques that may reduce the unfavorable effects of surgery. Tissue-engineered constructs are being developed to prevent attachment of scar tissue to the bone or as a substitute for the lacking mucosal tissue. A lot of in vitro research is also aimed at the elucidation of aspects of the oral wound healing process.
14.4.1 Effects of Surgery on Growth
Since suitable animal models for congenital clefts are not available, the effects of reconstructive surgery are evaluated with surgically created clefts in dogs, rabbits, and rats. Two different approaches have been used to evaluate the effects of surgery on midfacial growth and development of the dentition. The first approach is to create a cleft in the soft tissue and the palatal bone by surgical means. This cleft is subsequently closed again as the actual experimental intervention. It is obvious, however, that a surgically created cleft is different from a congenital one. Such a cleft creates a surgical trauma that might act as a confounding factor for the interpretation of the results. Only if the bony cleft is considered to be essential for evoking the disturbances in growth and development does this approach make sense. Bardach and coworkers have used this model since 1975. They performed some of the earliest experiments on the possible negative effect of lip repair (Bardach 1989, 1990; Bardach and Eisbach 1977; Bardach et al. 1979, 1980, 1993). Lip repair in rabbits and beagle dogs with surgically created complete unilateral clefts was found to result in a significant increase in lip pressure and a corresponding maxillary growth deficiency. The authors therefore suggested a causal relationship between the two.
The second approach is based on the assumption that the soft tissue intervention is crucial for the growth disturbances after cleft palate surgery. Already in the late 1960s, Kremenak and coworkers performed mucoperiosteal excisions without affecting the palatal bone in young beagle dogs as a model for the clinical situation after cleft palate repair (Kremenak et al. 1970). This approach led to growth disturbances that were similar to those after surgical cleft closure in children. Kremenak therefore concluded that “in this model, mucoperiosteal denudation of palatal shelf bone adjacent to deciduous molars is the single surgical variable responsible for the maxillary growth disturbances seen” (Kremenak et al. 1970). This is in agreement with later dog studies in which a midpalatal soft tissue cleft was created that was subsequently closed by von Langenbeck technique (e.g., (Wijdeveld et al. 1989, 1991)). Their results not only show the same effects on growth but also that the extent of this effect is related to the age at which surgery is performed. Growth disturbances turned out to be most prominent when surgery is performed before shedding of the deciduous dentition. Furthermore, these studies, as well as recent studies in rats (Kim et al. 2002), show that the deviations in maxillary arch dimensions are not only caused by a decreased sutural growth but also by a palatal tipping of the teeth in the lateral areas. In dogs, this tipping is especially prominent when surgery is performed at a young age, and it becomes apparent only after shedding of the deciduous dentition.
An explanation for this effect can be found in the healing of the soft tissue wounds and more specifically in wound contraction and scar tissue formation. Wound contraction in dog mucoperiosteum is most prominent in the first week after surgery. In palatal wounds in rats, it has been shown that the number of myofibroblasts increases considerably in that period (Cornelissen et al. 2000b), which also seems to be the case in dogs. Thereafter, the maturing granulation tissue is characterized by a gradual decrease in the number of fibroblasts and inflammatory cells and an increase in number and thickness of collagen type I fibers (Searls et al. 1979). Elastic fibers are not present in the granulation tissue or in the scar tissue at later stages (Wijdeveld et al. 1991).
A specific feature of the healing of open wounds in the mucoperiosteum is the deposition of callus-like cancellous bone on the palate. The granulation tissue adjacent to the palatal bone acquires an osteogenic potential, or osteogenic cells migrate into that area from adjacent periosteal tissues, and new bone is formed (Wijdeveld et al. 1991). This phenomenon is also known from other craniofacial bones and from long bones, where removal or mobilization of the periosteum leads to callus formation. Most of the collagen fibers of the scar are oriented in a transverse direction, but many fibers also show a vertical orientation. These vertical fibers become embedded in the cancellous palatal bone as Sharpey’s fibers, generating a strong attachment of the scar tissue to the underlying palatal bone (Wijdeveld et al. 1991) (Fig. 14.3).
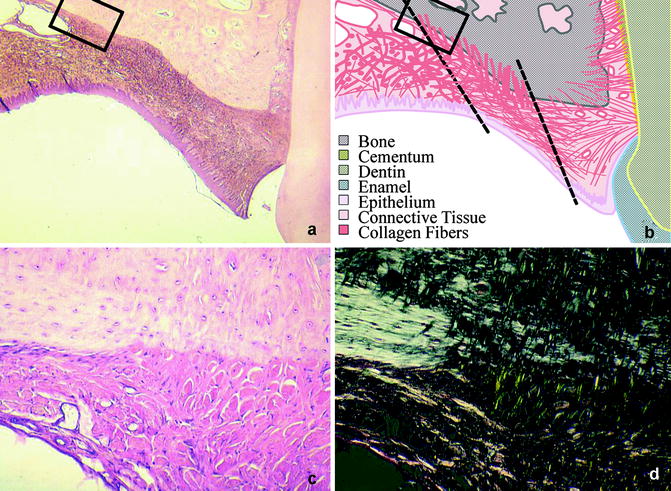

Fig. 14.3
(a–d) Palatal scar tissue. (a) shows the scar tissue adjacent to the teeth. The organization of the tissue is shown schematically in (b). (c, d) Shows an enlargement of the squared section. Note the thick perpendicular collagen fibers (Sharpey’s fibers) running into the bone, which are more clear when seen in polarized light (d)
The transverse fibers appear to be continuous with the cervical periodontal ligament, thus forming a mechanical connection between the teeth and the mucoperiosteal scar tissue (Kim et al. 2002; Wijdeveld et al. 1991). At the end of the growth period, the teeth alongside the scar tissue show a palatal tipping which is probably caused by the traction of the scar tissue on the erupting permanent dentition (Wijdeveld et al. 1988, 1989). These findings have led to the hypothesis that the iatrogenic effects of palatal surgery are initially caused by wound contraction, but scar tissue formation and the accompanying attachment of the scar tissue to the palatal bone and the teeth are probably the most important features. This leads to a restriction of maxillary growth and to a palatal tipping of erupting teeth in that region.
14.4.2 Modification of Surgical Techniques
Several researchers have tried to modify cleft palate surgery in order to avoid the appearance of denuded bone areas and the subsequent growth impairment. Perko was the first to use a mobilized mucosal split flap for palatal closure (Perko 1974). The disadvantage of his technique was the high risk of necrosis because the flap was only pedicled at the dorsal side. This technique has been modified by Leenstra et al. to obtain a flap that is pedicled both at the dorsal and the ventral side (Leenstra et al. 1995a, 1996). They used a partially split flap, leaving the lateral bone covered with the osteogenic layer of the mucoperiosteum, without impairment of the major neurovascular bundles (Leenstra et al. 1995a). In dogs, this technique was promising, since it led to less attachment of the mucoperiosteum to the underlying bone, and an improved transversal growth and development of the dentition (Leenstra et al. 1995a, b), and also in a clinical setting, the results were promising (Leenstra et al. 1996).
The denuded bone can also be covered with a biomaterial, which can either be applied as such or supplemented with cultured cells. This type of approach belongs to the field of tissue engineering.
14.4.3 Tissue Engineering
Tissue engineering has been defined in the late 1980s as “the application of principles and methods of engineering and life sciences toward fundamental understanding of structure-function relationships in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain or improve tissue functions” (Skalak and Fox 1988).
With respect to cleft palate surgery, a variety of approaches have been chosen to improve the outcome of the wound healing process. Firstly, biocompatible membranes have been used to prevent attachment of the scar tissue to the palatal bone or to reduce contraction and scar formation. A second approach is the engineering of mucosal substitutes to replenish the tissue defects. To this end, thin layers of keratinocytes have been cultured that can be used as epithelial grafts. Alternatively, keratinocytes have been cultured on top of a dermal substrate to produce a bilayered or composite graft, which is a substitute for the entire mucosa. The dermal substrate may consist of a collagenous matrix without cells or a matrix with cultured fibroblasts.
14.4.3.1 Biocompatible Membranes
Biocompatible synthetic membranes have been used to inhibit the attachment of scar tissue to the palatal bone by covering the denuded bone areas after surgery. Initially, the principles of guided tissue regeneration were used by inserting membranes in the mucoperiosteal defects (In de Braekt et al. 1992). These membranes were supposed to cover the palatal bone, inhibit osteogenic processes, and thereby prevent the formation of Sharpey’s fibers. Bio-resorbable Poly-L-lactic acid membranes and non-resorbable polymer membranes yielded unsatisfactory results. This was caused by an uncontrollable degradation of the lactic acid membranes and exfoliation or incomplete coverage of the bone by the non-resorbable membranes (Leenstra et al. 1998).
Next to synthetic membranes, collagen-based membranes have been used for intraoral surgery. Atelocollagen membranes have been successfully used to improve gingival healing in a rat model (Minabe et al. 1989). Similar membranes were used later in a model for cleft palate repair in young rabbits (Fujioka and Fujii 1997). In a split-mouth design, the membranes were implanted on the denuded palatal bone at the experimental side, while the control side was left open. The authors report that implantation reduced contraction and allowed more favorable growth of the palatal bone and normal development of the dentition.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


