This chapter explains human facial morphogenesis in an attempt to establish a framework for understanding the genesis of orofacial clefts. To facilitate this, illustrations created in the 1940s based on 3D reconstructions of histologic sections of human embryos as well as more recent scanning electron micrographs are presented and described. These images show the complex morphology of the developing human face, an appreciation of which is essential to interpreting experimental and clinical data regarding the genesis of both commonly occurring and unusual orofacial clefts. Of note, a majority of the multiple cleft sites, as defined by Tessier, correspond to the interface of morphologically distinct regions or growth centers of the embryonic face and can be readily related to failures of their growth, fusion, or merging.
Extensive discussion of the contributions and interactions of various cell types to yield the facial growth centers and result in their appropriate development is beyond the scope of this chapter. For this information, the reader is encouraged to consult recent reviews. Furthermore, this chapter is not intended to provide a comprehensive survey of known genetic factors and environmental influences that shape the face and can result in clefts. With knowledge in this arena growing at a rapid rate, reference to both recent reviews and databases of teratogens, genes, genetic disorders, and gene expression patterns for human beings and pertinent animal models is recommended (e.g., Online Mendelian Inheritance in Man: , Edinburgh Mouse Atlas Project: http://genex.hgu.mrc.ac.uk , and Mouse Genome Informatics: http://www.informatics.jax.org ).
For obvious reasons, only a minute portion of existing knowledge regarding normal and abnormal craniofacial embryogenesis is derived from examination of human embryos. Therefore extrapolations from descriptive and experimental studies of a broad spectrum of mammalian and nonmammalian species have been, and continue to be, common. Avian and rodent models have proven to be particularly informative. In this chapter the orofacial morphogenesis of these two species is compared with that of the human being, providing supportive evidence and new clues regarding the developmental basis for a variety of clefts.
COMPLEXITY OF HUMAN FACIAL MORPHOGENESIS
The developing face is most commonly described as being composed of tissues associated with the frontonasal prominence and the bilateral first pharyngeal (also known as visceral or branchial) arches. The frontonasal prominence surrounds the forebrain, and from it bilateral medial and lateral nasal prominences (also known as swellings or processes) form. The forehead, nose, philtral component of the upper lip, a portion of the upper alveolar ridge, and the primary palate develop from these portions of the embryo. The remainder of the face typically is described as arising from the first pharyngeal arches, each of which is composed of a maxillary prominence that forms the upper jaw and a mandibular prominence from which the lower jaw is derived. As illustrated and discussed in this chapter, this rather simplistic description of embryonic facial morphology is not complete and offers an inadequate framework on which to interpret clinical findings and new developmental investigations.
Although typically oversimplified when referenced in texts, a key embryology resource regarding human facial embryogenesis is a 1948 publication by G.L. Streeter. Selected images from this source are reproduced in Figure 34-1 . These drawings, created by J.F. Didusch from reconstructions of histologic sections, show human embryos ranging from 32 to 44 days after conception. Streeter described the developing face as being composed of a number of facial swellings (prominences) that reflect centers of growth. As shown in Figure 34-1, A, the primordia of the maxilla and lower jaw are apparent as separate swellings before those forming on the frontonasal prominence, that is, those that surround the olfactory pits. Regarding the tissue from which the lower jaw forms, Streeter noted that in addition to a median groove, lateral and transverse grooves are present, indicating multiple growth centers for this region of the developing face. In addition to forming the lower jaw, tissues of the lateral and caudal surfaces of the mandibular bar (i.e., first pharyngeal arch) were noted to contribute to the formation of the external ear, in particular the tragus and crus. From the hyoid bar (i.e., the second pharyngeal arch), the helix and antitragus of the ear form ( Figures 34-1, B to F ).
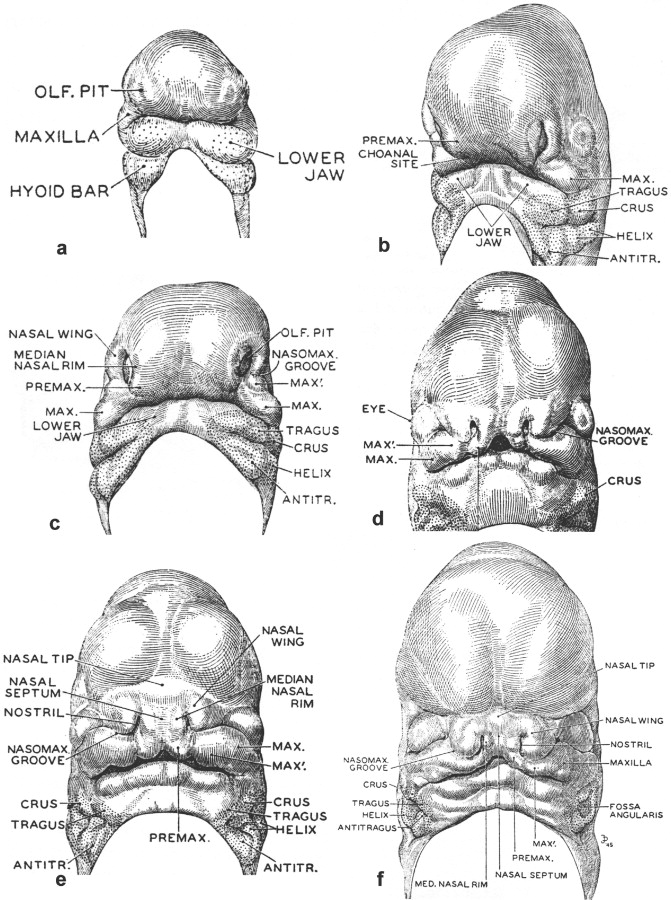
Streeter’s description of the growth centers that form the upper jaw include bilateral maxillary centers with a “characteristic subdivision” that he labeled MAX′ ( Figure 34-1, C to F ). Each of the MAX′ subdivisions is more rostrally positioned than the associated main maxillary centers (MAX) and are located adjacent to the developing frontonasal prominence. As the tissues of the frontonasal prominence develop, bilateral olfactory pits become apparent when growth centers termed the nasal wings (lateral nasal prominences [LNPs]) develop to form their dorsolateral boundaries, and medial nasal prominences (MNPs) arise at their medial rims. Growth centers located at the oral cavity (ventral) end of each medial nasal rim, but that are discrete from the nasal rim, are destined to form the premaxilla. Streeter noted that these growth centers could be termed the intermaxillary or incisive centers. They also are known as the globular processes of His. Most textbook descriptions currently do not distinguish the premaxillary (PREMAX) centers from the MNPs. Bilateral closure of the upper lip involves contact of the PREMAX with the MAX′ center and the ventral aspect of the LNP (LNP′) on each side of the developing face. Lip closure normally occurs during the sixth week of human gestation, when the embryo appears as shown in Figure 34-1, D and E.
Streeter’s work was extended in 1985 by Hinrichsen in a detailed report heavily based on scanning electron micrographs illustrating human facial morphogenesis. Figures 34-2 and 34-3 include four images from Hinrichsen’s work, with the remainder prepared by this author as part of the Necker collection (Necker Children’s Hospital, Paris, France). These scanning electron microscopic images of human embryos complement those of Didusch’s drawings, revealing a more complex array of facial growth centers than commonly recognized.
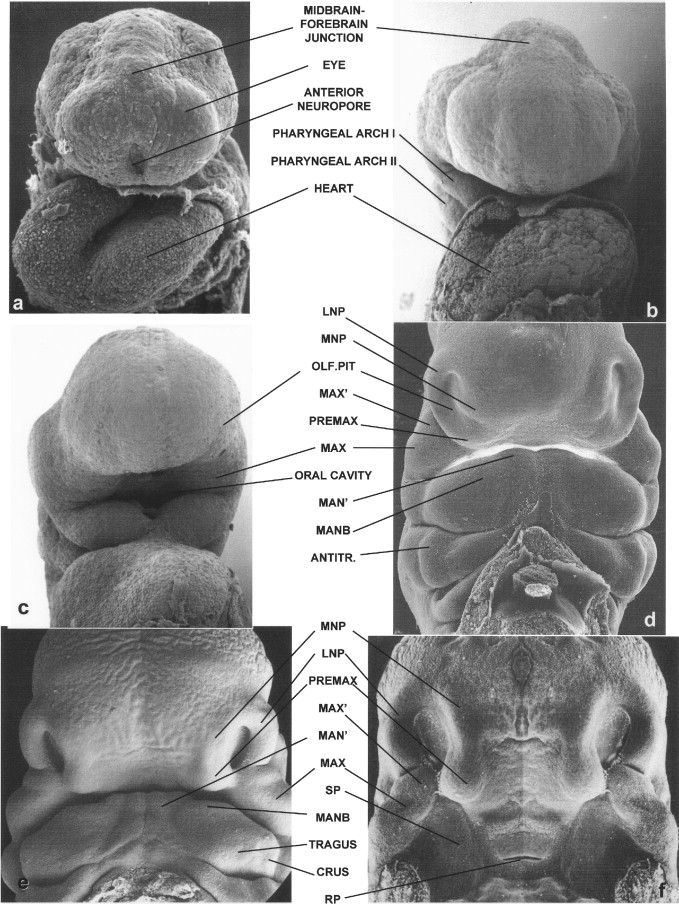
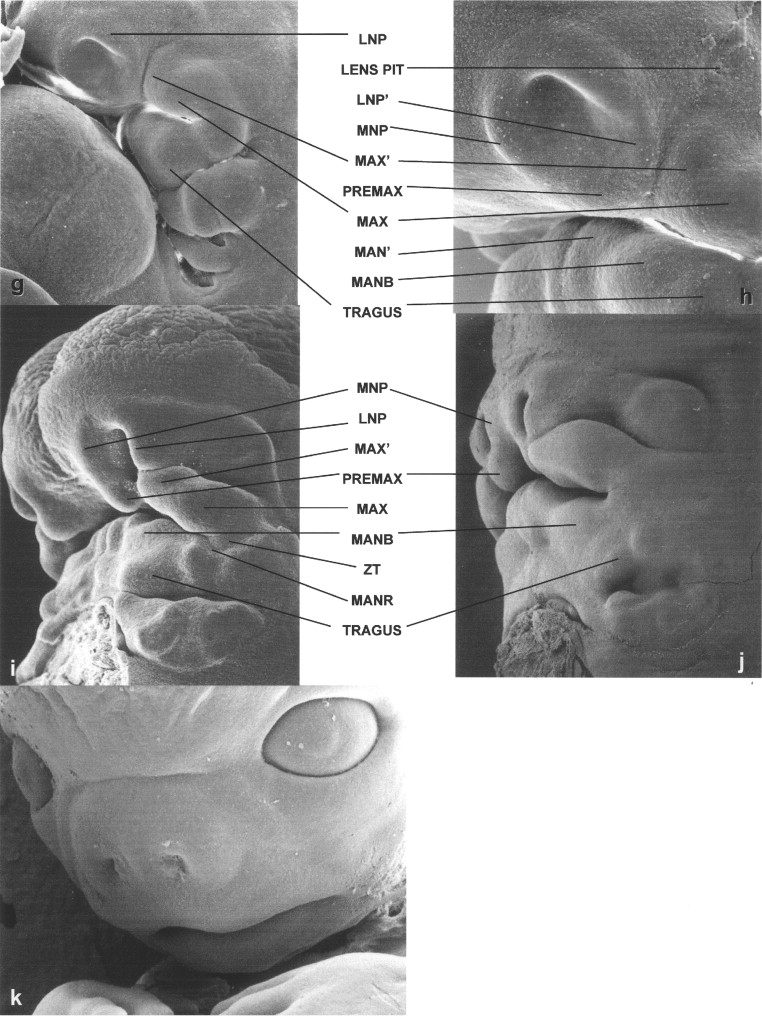
Micrographs in Figure 34-2 show frontal views of the faces of Carnegie stage 11 ( Figure 34-2, A ), 12 ( Figure 34-2, B and C ), 16 ( Figure 34-2, D and E ), 17 ( Figure 34-2, F and G ), and 18 embryos ( Figure 34-2, H and I ); stages present at 24 to 25, 26 to 27, 37 to 40, 41 to 43, and 44 to 46 days after fertilization, respectively. An image of a 9-week-old fetus also is shown ( Figure 34-2, J ). At the earliest stage shown, the anterior neuropore is completing its closure, a process that results in separation of the forebrain neuroepithelium from the surface epithelium of the frontonasal prominence ( Figure 34-2, A ). The developing eyes, which are evaginations from the forebrain, are seen at this time as bulges raised on each side of the developing upper midface. By 26 days, the site of the anterior neuropore is no longer apparent ( Figure 34-2, B ). Over the course of the next few days, the bilateral sites of the olfactory pits, around which the nose forms, become notable ( Figure 34-2, C; also see Figure 34-1, A ). As shown in Figure 34-2, C, the first pharyngeal arches, from which the lower jaw develops, initially have a deep median groove between them. By the time the embryo enters its sixth week of development, although a median groove is still present, the tissue bridge connecting the bilateral first arches is much wider ( Figure 34-2, D ).
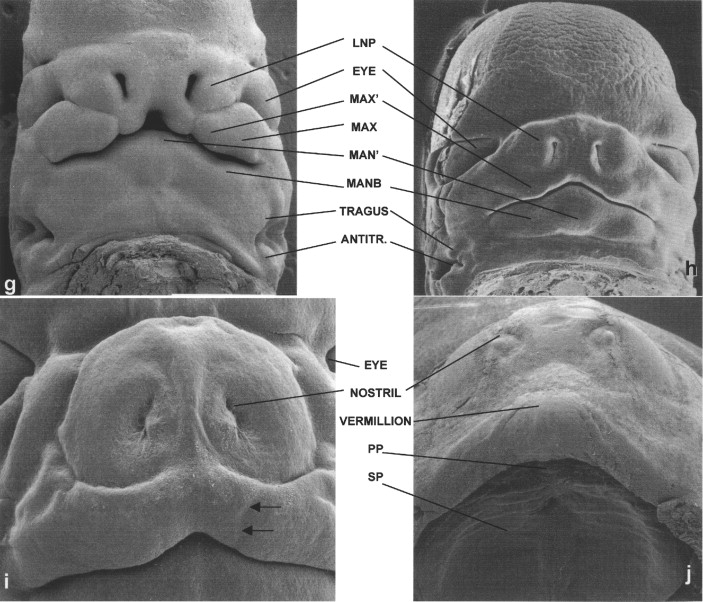
As also shown in Streeter’s work, in the developing lower jaw a shallow furrow separates the bulk of the first arch tissue from what appears to be a supplementary mandibular growth center that is the progenitor of the mentum. In this chapter, this tissue is termed MAN′. Another first arch growth center that can be identified at this time appears to correspond to the region of the future body of the mandible (MANB). Between 37 and 40 days of development, the first arch–derived external ear growth centers (auricular hillocks) become evident ( Figure 34-2, D and E ). Confirming Streeter’s findings, an obvious supplementary maxillary growth center (MAX′, Figure 34-2, D and F ) adjacent to the most ventral portion of the LNP (LNP″), as well as distinct MNP and PREMAX centers, also are present at this stage of development. On the side of MAX′ opposite LNP′ is the bulk of the MAX. Removal of the lower jaw, as has been done in the embryo shown in Figure 34-2, F, shows that a major portion of MAX extends into the oral cavity, forming the secondary palatal (SP) shelves. This view also allows visualization of Rathke’s pouch (RP), the progenitor of the anterior pituitary gland.
By the end of the sixth week, the lip should be closed bilaterally while a median groove between the PREMAX centers remains ( Figure 34-2, G ). Also by this time the six auricular hillocks from which both of the external ears form can all be seen. They comprise a large portion of the first and second pharyngeal arch tissue. Other prominent first arch components are MAN′ and the MANB. These mandibular subsegments also are apparent early in the seventh week of human gestation ( Figure 34-2, H ). At this time the PREMAX centers have merged in the midline. While the contour of the upper lip thus becomes smoother, at this stage in development the bilateral sites of union between the MAX′ and PREMAX remain apparent ( Figure 34-2, I ). By the beginning of the fetal period, epithelial plugs fill the nostrils, and the forming vermillion border of the upper lip is notable ( Figure 34-2, J ). In addition, the hard palate is closed as a result of the union of the SP shelves with each other as well as with the PREMAX-derived primary palate (PP).
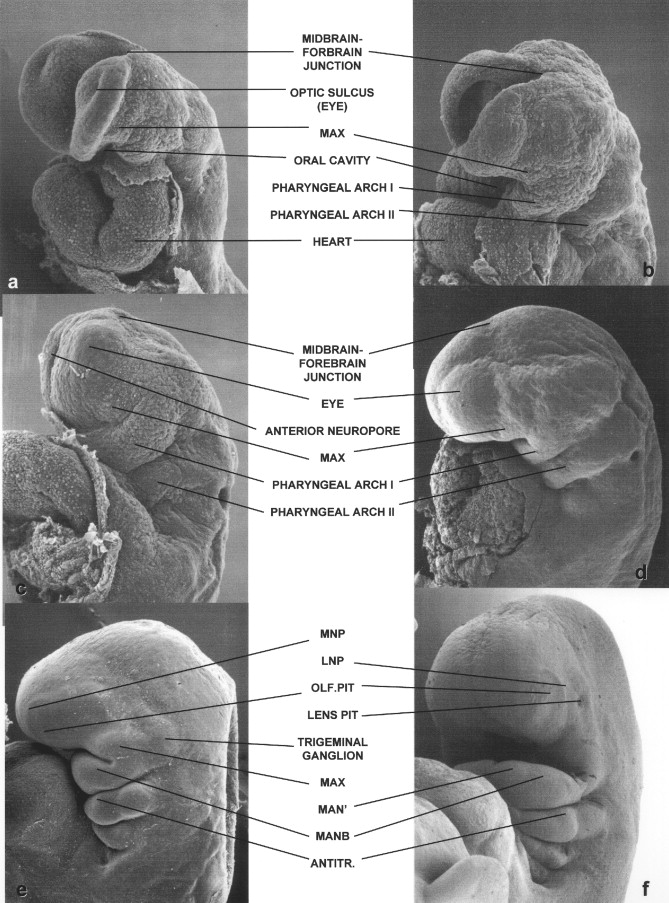
Scanning electron micrographs of the lateral aspects of the head and neck of a series of human embryos ranging from 24 to 53 days of gestation are shown in Figure 34-3 . From days 24 to 25 of development, the cranial end of the human neural tube completes its closure. This is evident in the three Carnegie stage 11 embryos shown in Figure 34-3, A to C. The embryo in Figure 34-3, A, still has a widely open forebrain; the rim of the forebrain of the embryo in Figure 34-3, B, has curved medially; and only a small anterior neuropore remains in the embryo shown in Figure 34-3, C.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


