Fractures of the facial skeleton are associated with cosmetic and functional deformities if left untreated or poorly managed. Each of the anatomic subunits of the face is susceptible to predictable wounding related to mechanisms of trauma. Trained specialists have historically anticipated facial fracture patterns and associated organ system injuries by understanding the mechanism of injury. Isolated orbital trauma has classically been associated with concentrated blunt and penetrating wound injuries. Orbital blow-out fractures should always be suspected when the victim reports diplopia and infraorbital nerve distribution numbness after receiving a concentrated direct blow to the eye. The adverse cosmetic and functional implications of severe enophthalmos, irrespective of mode of wounding, must be considered and prompt treatment initiated. As the world evolves, new threats have emerged to orbital and ocular health. Modernization and world conflict have introduced automobile air bag–related and terror-related eye injuries. Blast injuries following terrorist attacks are seen with increasing frequency worldwide. An increase of terror-related activities may require treatment of mass casualties that will necessitate a broadening of existing skills and knowledge of a variety of injury mechanisms.
Gunshot and explosion injuries caused by terrorist acts were characterized and compared in a retrospective cohort study of 1155 victims in Israel. Gunshot wound (GSW) patients suffered higher proportions of open wounds and fractures. Multiple body regions injured in a single patient occurred in 62% of explosion victims versus 47% in GSW patients. Overall, GSW and injuries from explosives differ in the body region of injury. Reports of blast injuries related to the Madrid terrorist train bombing attacks of March 11, 2004, identified orbital and paranasal sinus fractures among the most common injuries.
Children up to 14 years of age may be at risk for serious preventable injuries when seated in an automobile passenger seat equipped with an air bag. Current recommendations regarding children travelling in passenger vehicles equipped with passenger air bags are based on age and size. A total of 3790 patients (from birth to 18 years of age, seated in the right front passenger seat) were included in the National Automotive Sampling System (NASS) Crashworthiness Data System from 1995 to 2002. Sixty children (1.6%) were seriously injured. Among age, height, and weight, age from birth to 14 years (versus 15 to 18 years) was the only consistent effect modifier of the association between air bag presence (or air bag deployment) and serious injury, particularly for crashes with a moderate probability of injury. In a study of 150 orbital fractures diagnosed among 2739 occupants of motor vehicle crashes included in the Crash Injury Research and Engineering Network (CIREN) database from January 1997 to July 2005, 10 orbital blow-out fractures were attributed solely to air bag deployment. All crashes had air bag deployment and a frontal or near-frontal principal direction of force. Nine of 10 injured occupants were positioned within the air bag’s deployment zone at the time of impact as a result of forward seat track position, falling asleep at the wheel, being unrestrained, or having decelerated before impact. Six of 10 occupants were shorter than average height.
Air bags clearly contribute substantially to improving vehicle safety when combined with other safety measures, particularly seat belt restraints. The incidence and overall severity of orbital injuries are reported to decrease with exposure to air bag deployment. In what the authors describe as “the most comprehensive study of orbital fractures in automobile crashes to date,” the National Automotive Sampling System database files from 1993 to 2000 were examined. Frontal crashes were selected that included drivers and front-seat passengers only and excluded ejected occupants and rollover crashes. The analysis included 12,429,580 front-seat occupants from 25,464 cases. Of all occupants who were exposed to an air bag deployment, 0.09% sustained an orbital fracture. In contrast, occupants who were not exposed to an air bag deployment were more than twice as likely to sustain an orbital fracture (0.22%).
DIAGNOSIS AND CLASSIFICATION OF ORBITAL FRACTURES
ANATOMY
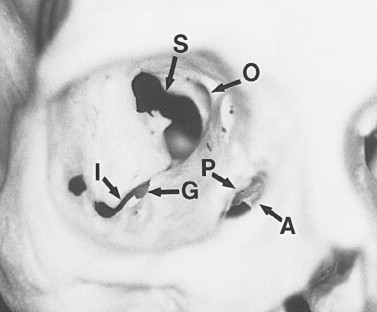
The orbit is a unique bony structure that has the primary purpose of housing and protecting the globe. By age 5 years, orbital growth is 85% complete; growth is finalized between 7 years of age and puberty. The orbit is a four-sided pyramid with its apex at the optic foramen; anteriorly, its base is formed by the orbital rim ( Figure 13-1 ). The orbital rim consists of dense cortical bone that protects the orbital contents from direct trauma. Seven bones contribute to the formation of the orbit: maxillary, zygomatic, frontal, ethmoidal, lacrimal, palatine, and sphenoid. These bones form a socket for the globe, provide insertions for the extraocular muscles, and are intimately associated with the surrounding sinuses and cranial fossae. Numerous nerves and vessels course through foramina, fissures, and canals of the orbital bones ( Table 13-1 ).
| Location | Contents | |
|---|---|---|
| Optic canal | Lesser wing of sphenoid | Optic nerve, meninges, ophthalmic artery sympathetic fibers |
| Superior orbital fissure | Lesser and greater wings of sphenoid | |
| Inferior orbital fissure | Greater wing of sphenoid; palatine, zygomatic, and maxillary bones |
|
| Anterior ethmoid canal | Frontal and ethmoid bones | Nerve: anterior ethmoid becomes dorsal artery |
| Posterior ethmoid canal | Frontal and ethmoid bones |
|
| Nasolacrimal fossa | Lacrimal and maxillary bones | Nasolacrimal sac and duct |
* Enters the orbit outside the annulus of Zinn and muscular cone.
The orbital walls vary in thickness and strength. Fractures of the anterior and middle thirds of the orbit are common, with the maxillary sinuses and ethmoid air cells serving as shock absorbers and acute volume expansion compartments. As a result, globe perforation and acutely increased intraocular pressure (>20 to 25 mm Hg) are relatively infrequent following midfacial trauma. Except in severe life-threatening trauma, fractures occurring in the posterior one third of the orbit are rare. It is these fractures that can more commonly result in blindness.
The roof of the orbit consists largely of the orbital plate of the frontal bone, with the lesser wing of the sphenoid contributing only a minor portion posteriorly. The roof of the orbit is thin and separates the orbit from the anterior cranial fossa. In the elderly, the orbital roof may become resorbed, resulting in areas where the periorbita becomes fused to the overlying dura. The anterior portion of the roof is occupied by the supraorbital extension of the frontal sinus. Anterolaterally is a smooth, broad fossa for the lacrimal gland. Medially, approximately 4 mm behind the rim, lies the trochlear fovea, where the cartilaginous pulley inserts for the superior oblique muscle tendon. At the junction of the medial one third and lateral two thirds of the superior rim lies the supraorbital notch, which is often converted to a foramen (in 25% of individuals) by ossification of the ligament crossing it, thus encasing the supraorbital nerve and vessels.
The thin orbital floor is defined posterolaterally by the sphenomaxillary fissure. However, there is no distinct border medially. The bones of the floor consist of the orbital process of the maxilla; anterolaterally, a portion of the zygomatic bone; and posteriorly, a small portion of the palatine bone. The infraorbital groove originates from the middle of the infraorbital fissure approximately 2.5 to 3 cm from the infraorbital rim and converts to a canal halfway forward. The infraorbital groove and canal carry the infraorbital nerve and vessels, which innervate the soft tissues of the upper lip, the mucosa over the anterior maxilla, and the anterior maxillary teeth. The canal opens 5 mm below the rim of the maxilla as the infraorbital foramen. The floor of the orbit is often only 0.5 mm thick and separates the maxillary sinus from the orbital contents. The thinnest portion of the floor is just medial to the infraorbital groove and canal.
The lateral wall of the orbit is composed of the greater wing of the sphenoid bone and the frontal process of the zygoma. This is the strongest wall, but it may be fractured along its thinnest portion of the suture line, where the greater wing of the sphenoid and zygoma join. This wall separates the orbit from the temporalis muscle. The lateral wall is angled 45 degrees with respect to the medial wall and 90 degrees with respect to its other counterpart. The lateral wall and roof of the orbit, as well as the greater and lesser wing of the sphenoid, are separated by the superior orbital fissure. At the orbital apex, the lesser wing of the sphenoid forms the lateral wing of the optic canal. Internally, just behind (5 mm) the lateral orbital rim, is Whitnall’s tubercle. The tubercle is approximately 1 cm below the frontozygomatic suture. This slight projection functions as the attachment for lateral retinacular structures, namely, the lateral horn of the levator aponeurosis, lateral canthal tendon, inferior suspensory (Lockwood’s) ligament, and check ligament of the lateral rectus muscle. These soft tissue attachments are found anatomically in this order as one proceeds inferiorly and posteriorly, and they become confluent to form the lateral retinaculum, which actually attaches to the tubercle.
The medial wall of the orbit is by far the most complex. Arranged anteriorly to posteriorly, it consists of a portion of the frontal, lacrimal, ethmoid, and sphenoid bones. The maxilla contributes slightly at the anterior and inferior boundaries. The orbital surface of the ethmoid, or lamina papyracea , is extremely thin (0.2 to 0.4 mm) and forms the largest section of the medial wall. At the most anterior extent of the medial wall is the anterior lacrimal crest, which is formed by the frontal process of the maxilla. Proceeding posteriorly, the lacrimal fossa is then defined by the posterior lacrimal crest, a part of the lacrimal bone. Approximately 20 to 25 mm behind the medial orbital rim is the anterior ethmoidal foramen, and 12 mm further back is the posterior ethmoidal foramen. These foramina appear two thirds of the way up the medial wall within the frontoethmoidal suture and mark the level of the cribriform plate. The anterior ethmoidal foramen contains the anterior ethmoidal artery and anterior ethmoidal branches from the nasociliary nerve. The posterior ethmoidal foramen transmits the posterior ethmoidal artery and inconsistently a sphenoethmoidal nerve from the nasociliary nerve.
The surgeon must be aware of the limits of safe subperiosteal dissection ( Figure 13-2 ). A subperiosteal dissection can be safely extended 25 mm posteriorly from the inferior and lateral rims. An exploration distance of 30 mm from the superior orbital border or anterior lacrimal crest can be performed. The surgeon must bear in mind that these safe distances are referenced from intact adult orbital rims. When a traumatic force has displaced a portion of the rim, it is generally in a direction (posterior and medial) that effectively reduces these distances. At all times the surgeon must be aware of the orbital bony anatomy and the vital structures that course through it. Care must be taken to avoid disruption of the medial canthal tendon, lacrimal apparatus, and pulley of the superior oblique muscle; supraorbital nerves and vessels; structures attached to Whitnall’s tubercle; and the origin of the inferior oblique muscle. Rontal and colleagues arrived at mean distances for locating vital structures in relationship to identifiable fine bony landmarks ( Table 13-2 ).
| Reference Landmark | Structure | Mean Distance (mm) |
|---|---|---|
| Infraorbital foramen | Midpoint of inferior orbital fissure | 24 |
| Anterior lacrimal crest | Anterior ethmoidal foramen | 24 |
| Anterior lacrimal crest | Optic canal (medial aspect) | 42 |
| Zygomaticofrontal suture | Superior orbital fissure | 35 |
| Supraorbital notch | Superior orbital fissure | 40 |
| Supraorbital notch | Optic canal (superior aspect) | 45 |
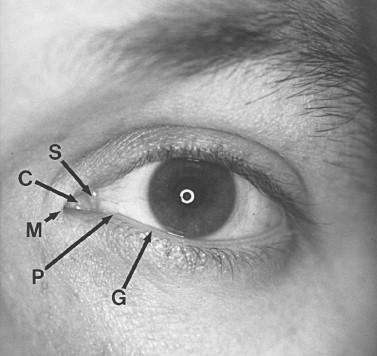
The eyelids and fibrous orbital septum form the anterior boundary of the orbit. The upper and lower eyelids are similar in anatomic composition, except for the lid retractors, so they are discussed jointly ( Figure 13-3 ). The lids are surfaced by a very thin keratinized epithelium. The skin is loosely attached to the orbicularis oculi muscle ( Box 13-1 ), which is innervated by cranial nerve VII. The closing action of the orbicularis oculi muscle is chiefly opposed in the upper lid by the levator palpebrae superioris, which is innervated by cranial nerve III. The resting tone of the upper eyelid is partly determined by the amount of sympathetic input to Müller’s muscle. The orbicularis oculi is divided into the orbital portion, outer superficial fibers, and the palpebral section (central most deep fibers). The palpebral section medially has intricate insertions and envelops the lacrimal sac by dividing into intertwined deep and superficial heads. The superficial heads insert into the medial canthal ligament; the deep heads insert into the fascia of the lacrimal sac and posterior lacrimal crest. The medial canthal tendon or ligament is formed by the confluence of thickened fibrous insertions. The medial canthal tendon has a thin, deep head that inserts into the posterior lacrimal crest and a thick, superficial head that tenaciously inserts into the anterior lacrimal crest. Laterally, the more superficial fibers of the orbicularis oculi form an indistinct raphe, and the deeper fibers insert as the lateral canthal tendon onto Whitnall’s tubercle. The lids form a 30- to 40-degree angle at the lateral canthus, usually 1 cm below the frontozygomatic suture. The lateral canthus is usually 2 mm above the level of the medial canthus.
-
Skin
-
Subcutaneous areolar tissue
-
Striated muscle (orbicularis oculi)
-
Submuscular areolar tissue (contains main sensory nerves to lids)
-
Fibrous layer with tarsal plates
-
Nonstriated smooth muscle
-
Mucous membrane or conjunctiva
Deep to the orbicularis oculi is the fibrous orbital septum, which is confluent with the periorbita (orbital periosteum) and the periosteum of the facial bones composing the rims. In the lower lids, this junction is thickened and is termed the arcus marginale . It is located 1 to 2 mm below the edge of the inferior rim. At the distal edges, the orbital septum is confluent and inserts into the underlying levator aponeurosis (upper lid) and the capsulopalpebral fascia (lower lid). Preaponeurotic orbital fat is retained by the septum in both lids and is divided into several pads above (central and medial) and three below (medial, central, and lateral). With aging and increased connective tissue laxity of the orbital septum, these preaponeurotic orbital fat pads may bulge externally, creating “baggy lids.” Severe sagging of the lower lids is referred to as “festooning.”
The levator palpebrae superioris muscle (primary elevator of the upper lid) consists anteriorly of an aponeurosis that attaches broadly over the superficial anterior two thirds of the tarsal plate and subcuticular tissue. Approximately 15 to 20 mm above the tarsal plate, the aponeurosis consists of a fascial condensation called Whitnall’s ligament. This ligament acts as a suspensory ligament of the globe. Müller’s muscle arises beneath Whitnall’s suspensory ligament. Müller’s muscle is a smooth muscle that relies on sympathetic input for its tone and regulates the resting position of the upper lids while the eyes are open. The lower lid retractors are known as the capsulopalpebral fasciae, which extend from the inferior rectus and inferior oblique muscles to fuse with the inferior suspensory ligament of Lockwood. The tarsal plate is dense, fibrous tissue that largely maintains the convex form of each lid. The border of the tarsus is next to the free margin of each lid. The length of each tarsus is approximately 45 mm, and its width and thickness are greatest midway on the lid. The width of the upper tarsus (11 mm) is about twice that of the lower (5 to 6 mm). Within the tarsal plates are embedded many slender meibomian (sebaceous) glands. When these glands become chronically obstructed and inflamed, they form a cystlike mass termed a chalazion .
Tears or lacrimal secretions accumulate at the medial inferior aspect of the eye. The fluid is then picked up by the lacrimal puncta, which are two small (0.2 to 0.3 mm in diameter) openings in the upper and lower lids. The upper punctum is usually slightly medial in relation to the lower punctum ( Figure 13-4 ). The upper and lower canaliculi travel within the lids first vertically (2 mm), and then horizontally, paralleling the lid margin for 8 to 10 mm, where they join to form the common canaliculus (3 to 5 mm). The common canaliculus enters the lacrimal sac on the lateral aspect of the junction of the upper and middle thirds. The lacrimal sac, which is approximately 1 cm in length and 5 mm in diameter, is encased by a dense, adherent fascia. The nasolacrimal duct has a 12-mm intrabony canal and eventually opens below the inferior turbinate into the inferior meatus, usually 30 to 35 mm from the external nares. At the opening of the duct is a mucosal fold called the valve of Hasner. This valve helps prevent fluid and air reflux when high intranasal pressure is generated by blowing one’s nose. With epiphora it is important to establish where the mechanical obstruction exists within the lacrimal drainage system. Canalicular irrigation may relieve temporary obstruction caused by dried, thickened secretions. A Jones’ dye test or dacryocystography can help determine the point of obstruction and thus guide surgical planning. Also, epiphora may be due to hypersecretion. Following trauma, corneal abrasion, a foreign body, lash ptosis, or entropion may serve as the stimulus for the lacrimal gland’s overproduction.
FRACTURE PATTERNS
Isolated orbital fractures constitute 4% to 16% of facial fractures. If one includes orbital fractures that extend outside the confines of the orbit into the facial skeleton, the incidence approaches 30% to 55%. Fractures involving the orbit can be classified as zygomatic complex, naso-orbital-ethmoid (NOE), or internal orbital fractures.
Zygomatic complex fractures are the most commonly occurring facial fracture, second only to nasal fractures. These fractures are also the most commonly occurring fractures of the orbit. Zygomatic complex fractures are often displaced in an inferior, medial, and posterior direction, and the complex fulcrums about the frontozygomatic suture. Various classification systems have been reported; however, the system proposed by Jackson is most notable ( Figure 13-5 ). It classifies the fracture based on severity: type I (nondisplaced), type II (segmental fracture of the orbital rim), type III (tripod fracture), and type IV (fragmented).
NOE fractures most often occur following blunt trauma to the midface. These fractures result primarily in cosmetic deformities such as flattening of the nasal dorsum and widening of the intercanthal distance. This can occur as a result of disruption of one or both medial canthal ligaments. Lacrimal drainage problems can also arise from NOE fractures. The severity of these fractures can range from minimal displacement of a large segment of the medial orbital rim to severe comminution with complete avulsion of the canthal ligament from the bone ( Figure 13-6 ).
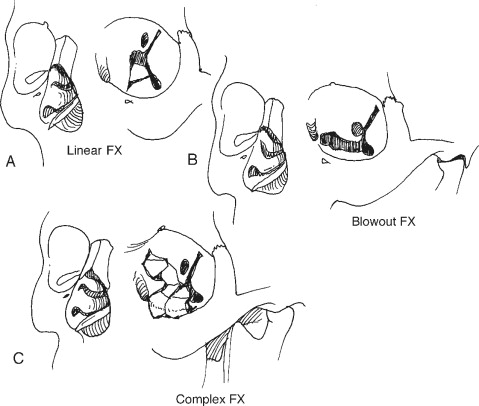
Internal orbital fractures can occur in numerous patterns. They are often described by their location and the size of the defect. Three patterns of internal orbital fractures have been described : linear, blow-out, and complex ( Figure 13-7 ). Linear fractures maintain periosteal attachments and therefore do not result in a defect; however, they can result in significant enlargement of the orbit. Blow-out fractures are the most commonly occurring injury and are limited to one wall, with a defect of less than 2 cm in diameter. Blow-out fractures most commonly occur in the anterior or middle part of the floor; however, they can also occur in the medial or superior walls, where they can present as blow-in fractures ( Figure 13-8 ). Exploration, repair, and/or reconstruction of orbital roof fractures may be indicated if a dural tear is suspected or to prevent a “pulsatile globe.” This inward and outward movement of the eye is due to arterial pulsations and respiratory fluctuations that occur in the overlying cerebral hemisphere. This phenomenon often manifests after resolution of edema and hematoma in unrepaired roof defects and causes persistent blurred or double vision. Complex fractures consist of extensive fractures that affect two or more orbital walls; they often involve the posterior orbit and may involve the optic canal. Complex fractures are usually associated with fractures of the facial skeleton outside the orbital frame (LeFort II and III fractures or frontal sinus fractures), which are classified as combined fractures.
CLINICAL EXAMINATION
A brief history of the mechanism of injury and direction of force should be ascertained before performing a clinical examination of the orbit and globe. A systematic approach assessing both orbits will further define functional and anatomic defects associated with orbital injuries. The initial ophthalmologic evaluation should include periorbital examination, visual acuity, ocular motility, pupillary responses, visual fields, and funduscopic examination.
Visual acuity is independently tested for each eye using a Snellen chart at a standard 20-foot distance or a near card (standard-type print at 14 inches) if a Snellen chart is unavailable. If the patient wears corrective lenses, these should be worn during the examination.
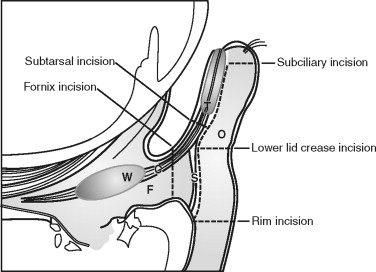
The eyelids and periorbital area should be inspected for edema, ecchymosis, lacerations, ptosis, asymmetric lid drape, canalicular injury, and canthal tendon disruption. When traumatic forces have produced lid ecchymosis, there should be heightened awareness of the possibility of an occult ocular injury or an orbital wall fracture. A lid retractor (Desmarres) is useful in separating edematous eyelids to examine the globe and can be used to inspect the inner aspect of the lid. Upper lid lacerations with fat herniations below the level of the brow should alert the examiner to potential full-thickness lid injury or underlying penetrating globe injury ( Figure 13-9 ). If the pleural conjunctiva has been violated, it is prudent to consult an ophthalmologist to assess for ocular penetration. Gentle lateral traction on the lower lids can reveal medial canthal disruption.
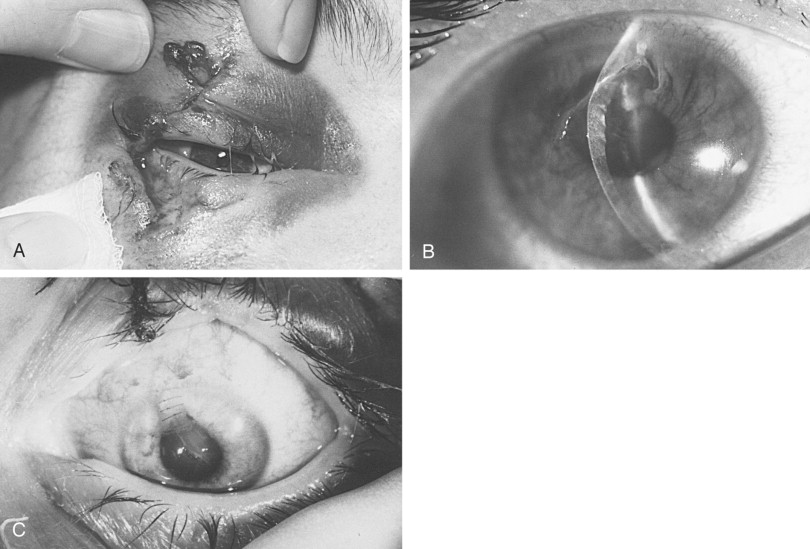
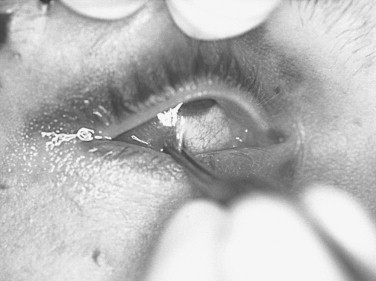
Extraocular movements are evaluated to rule out mechanical entrapment or paresis. Any diplopia and the field of gaze in which it occurs should be noted. Mild (<5 degrees) restriction in extreme fields of gaze is common in the face of severe orbital edema. After topically anesthetizing the eye, forced ductions with fine-toothed forceps can be performed in the direction of limitation to determine a mechanical (entrapment) versus a neurologic origin ( Figure 13-10 ).
Pupillary size, shape, and symmetry should be evaluated, as well as light reactivity. In any patient with anisocoria (unequal pupils) or an irregular pointing pupil, a history of previous ocular trauma or eye surgery (cataract) should be elicited. An irregular pupil often points toward the site of globe penetration or injury (see Figure 13-9, B ). The globe should also be evaluated for acute enophthalmos or proptosis, which can be ascertained by viewing directly from above or below. In addition, the vertical position of the globe should be noted.
Visual fields are tested for each eye, one at a time, by confrontation. This involves directly aligning the patient’s and examiner’s faces 2 feet apart (both maintaining direct forward gaze) and asking the patient to detect movement at the extremes of the examiner’s own visual field. Quadratic defects may indicate ocular, optic nerve, or optic pathway damage.
A funduscopic examination should be performed in a dimly lit room. Lens dislocation, vitreous hemorrhage, retinal hemorrhage, retinal detachment, and foreign bodies can be noted. If history and initial findings warrant a dilated funduscopic examination, neurologic status and clearance should first be obtained. An ophthalmologist is best qualified to perform a dilated funduscopic examination, searching for more occult ocular injuries. Also, when the history or initial examination warrants further evaluation, tonometry and a slit-lamp examination can be performed, usually by an ophthalmologist.
Tonometry measures intraocular pressure in both eyes by surface readings. Although normal (10 to 20 mm Hg) or equal readings are reassuring, they do not rule out a penetrating injury. A relatively low pressure reading in an injured eye suggests scleral rupture. An abnormally high or unequal reading may be indicative of a retrobulbar hematoma, which may require acute evacuation via a lateral canthotomy.
A slit-lamp examination is performed with the patient in an upright position. If the patient is confined to bed, a modified examination with a penlight can be performed. The examiner can rule out conjunctival chemosis, hemorrhage, emphysema, and foreign bodies. Fluorescein dye and a cobalt blue light (Wood’s lamp) facilitate corneal assessment for abrasion, laceration, and foreign body. The anterior chamber is assessed for depth, clarity, and hyphema (blood in the anterior chamber). The shape and reactivity of the iris are also noted.
Last, the bony orbital rim should be palpated for evidence of a bony step, bony crepitus, or mobility. Neurologic sensory testing can also be performed to evaluate the supraorbital, supratrochlear, and infraorbital nerves. Once a complete ophthalmologic examination has been performed, additional studies such as a computed tomography (CT) scan or magnetic resonance imaging (MRI) can be selectively ordered, with meaningful results.
IMAGING TECHNIQUES
Imaging is essential for the proper diagnosis and treatment of orbital trauma. Noncontrasted CT is the primary imaging modality currently used for evaluating injuries related to blunt and penetrating orbital trauma, as well as for localizing most orbital foreign bodies. Secondary modalities such as plain radiography, three-dimensional CT scan, MRI, ophthalmic echography, color Doppler imaging, and angiography may provide useful supplemental data in certain instances.
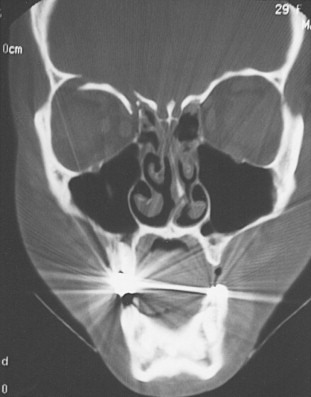
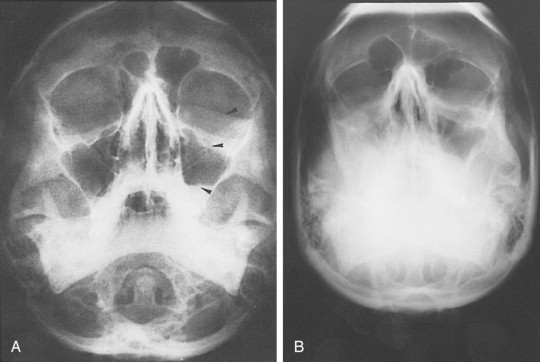
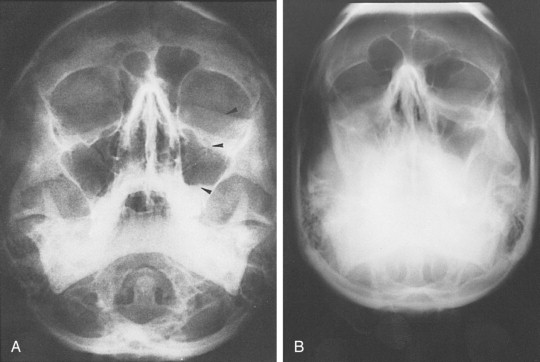
Standard radiographs are readily available and provide a relatively inexpensive method of assessing external orbital fractures. They are, however, inadequate in evaluating internal orbital fractures and soft tissues. Foreign bodies can be detected on plain films, but localization is difficult. Several views may be useful in evaluating orbital injury. Caldwell’s projection provides a good view of the superior and lateral orbital rim, the medial wall, and the ethmoidal and frontal sinuses. Waters’ projection allows visualization of the orbital roof and floor and is useful when evaluating blow-out fractures ( Figure 13-11 ). Lateral projections may be used to study the floor and posterolateral orbital wall. Basal and oblique projections may be obtained to evaluate the optic canal.
CT has replaced standard radiographs and conventional tomography as the optimal technique to diagnose fractures of the orbit. CT allows excellent visualization of orbital soft tissues as well as the ability to simultaneously assess the intracranial cavity when evaluating facial and orbital trauma. Fractures are best evaluated when the imaging plane is perpendicular to the fracture line. As a result, images are usually obtained in two different planes unless a known injury is being evaluated. The standard imaging approach for facial trauma is to obtain direct (not reformatted) 3-mm sections in the axial and coronal planes, without intravenous (IV) contrast. Coronal sections are preferred for evaluating orbital roof and floor fractures ( Figure 13-12 ). If an optic canal fracture is suspected, 1.5-mm axial cuts should be obtained. By obtaining both views, the specific number and location of fractures can be determined. Soft tissue injuries such as lens dislocation, vitreous hemorrhage, ruptured globe, retrobulbar hemorrhage, or avulsion of the optic nerve are readily demonstrated by CT. CT is also the modality of choice in detecting and localizing most metallic and nonmetallic foreign bodies in relation to the globe, extraocular muscles, and optic nerve. The extent and size of subperiosteal hematomas may also be delineated with CT images.
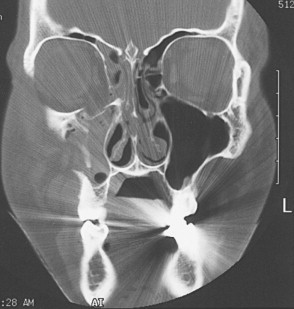
Patients with cervical spine or severe head injuries cannot be imaged in the direct coronal plane. In these patients, computer-reformatted coronal images can be obtained based on the axial image set. There is, however, loss of spatial resolution with computer-reformatted images. Also, the axial image set must be obtained with thin slices (1 to 3 mm). Small amounts of patient motion will introduce artifacts into the reformatted images. Sagittal or semisagittal reformatted images may occasionally show better delineation of anterior and posterior fracture margins than coronal or axial views.
CT imaging is not without limitations. Coronal CT is uncomfortable and often impossible to perform. Sedation is frequently required in pediatric patients. In these instances, the risk of the procedure versus the potential benefits must be weighed. The cost of CT scanning is higher than the cost of standard radiographs. Radiolucent foreign bodies such as wood are often missed on CT scan. In certain cases, echography and MRI are helpful in detecting radiolucent foreign bodies when CT scans are equivocal or negative.
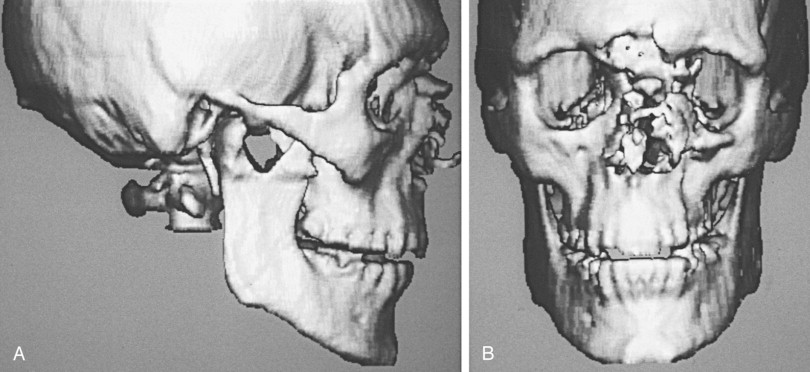
Computer-generated three-dimensional CT images may provide improved depiction of complex orbital facial fractures ( Figure 13-13 ). They generate more spatial information and show fracture extent and fragment displacement in severe trauma with multiple fracture fragments. In these instances, three-dimensional CT can be a useful adjunct in surgical planning. Patients must remain absolutely motionless during the scan, which often requires over 100 axial slices to obtain adequate results. Thin fracture lines are, however, usually invisible on three-dimensional CT.
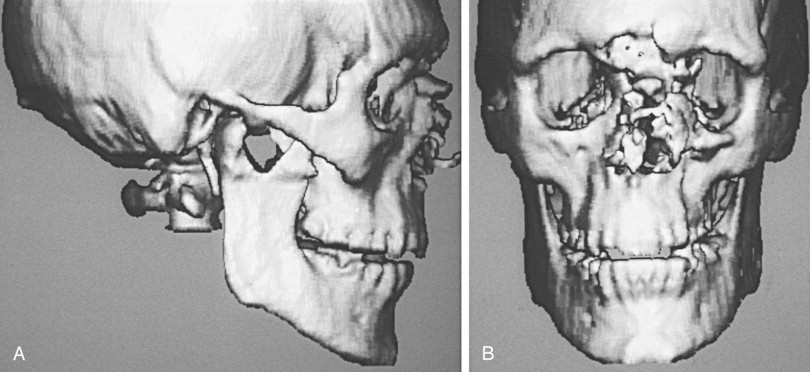
MRI has limited uses in orbital trauma and is less sensitive than CT in depicting fractures. MRI is useful for assessing soft tissue involvement, such as incarceration of extraocular muscles or orbital fat. It may be useful in evaluating orbital fractures if direct coronal CT is not obtainable or in cases of excessive dental artifact. Standard radiographs or CT scans should be obtained before MRI is performed on patients with suspected intraocular or intraorbital ferromagnetic bodies, because of the potential for displacement and significant ocular injury. Wood can appear isodense with fat and air on CT. This may result in misinterpretation of orbital scans. MRI has been used to identify wood and foreign bodies that have been misdiagnosed by CT scan. MRI should be considered when penetration of wooden foreign bodies is likely and when an apparent air collection within the post-traumatic orbit does not rapidly resorb, which suggests a space-occupying foreign body.
A correlation between ocular motility and evaluation of computed tomography in orbital blow-out fractures was retrospectively studied in 113 cases of pure blow-out fracture with diplopia. Patient’s satisfaction with treatment was based on percentage of Hess area ratio (HAR%) on the Hess chart. Fracture type and the number of points of contact of extraocular muscles to the fracture edge were recorded from CT findings. Initial CT findings of the rectus muscle’s contact with the fracture edge were strongly correlated with surgical outcome.
Postoperative ocular motility and residual diplopia were retrospectively studied in 30 patients with orbital blow-out fractures by correlating preoperative CT findings. The authors concluded that when the CT-depicted relationship between bone fragments and soft tissue is considered, greater degrees of soft tissue incarceration or displacement appear to result in poorer motility outcomes. An urgent surgical approach to selected orbital injuries was recommended.
Ophthalmic echography may be used occasionally to evaluate traumatic injuries of the orbit. It is a readily available, safe, inexpensive, noninvasive imaging modality. Foreign bodies located in the anterior orbit may be identified with ultrasonography; however, because of highly reflective orbital structures such as fat and bone, the foreign-body signal may be obscured when the object is located in the orbital apex. Wood and other radiolucent material that may not be visible on CT scan can be detected with ultrasonography. Color Doppler imaging (CDI) is a more recent ultrasound technique that gives simultaneous two-dimensional imaging of structure and blood flow. CDI is useful in evaluating post-traumatic high-flow carotid cavernous fistulae. However, angiography remains the definitive procedure in establishing the diagnosis.
IMAGING TECHNIQUES
Imaging is essential for the proper diagnosis and treatment of orbital trauma. Noncontrasted CT is the primary imaging modality currently used for evaluating injuries related to blunt and penetrating orbital trauma, as well as for localizing most orbital foreign bodies. Secondary modalities such as plain radiography, three-dimensional CT scan, MRI, ophthalmic echography, color Doppler imaging, and angiography may provide useful supplemental data in certain instances.
Standard radiographs are readily available and provide a relatively inexpensive method of assessing external orbital fractures. They are, however, inadequate in evaluating internal orbital fractures and soft tissues. Foreign bodies can be detected on plain films, but localization is difficult. Several views may be useful in evaluating orbital injury. Caldwell’s projection provides a good view of the superior and lateral orbital rim, the medial wall, and the ethmoidal and frontal sinuses. Waters’ projection allows visualization of the orbital roof and floor and is useful when evaluating blow-out fractures ( Figure 13-11 ). Lateral projections may be used to study the floor and posterolateral orbital wall. Basal and oblique projections may be obtained to evaluate the optic canal.
CT has replaced standard radiographs and conventional tomography as the optimal technique to diagnose fractures of the orbit. CT allows excellent visualization of orbital soft tissues as well as the ability to simultaneously assess the intracranial cavity when evaluating facial and orbital trauma. Fractures are best evaluated when the imaging plane is perpendicular to the fracture line. As a result, images are usually obtained in two different planes unless a known injury is being evaluated. The standard imaging approach for facial trauma is to obtain direct (not reformatted) 3-mm sections in the axial and coronal planes, without intravenous (IV) contrast. Coronal sections are preferred for evaluating orbital roof and floor fractures ( Figure 13-12 ). If an optic canal fracture is suspected, 1.5-mm axial cuts should be obtained. By obtaining both views, the specific number and location of fractures can be determined. Soft tissue injuries such as lens dislocation, vitreous hemorrhage, ruptured globe, retrobulbar hemorrhage, or avulsion of the optic nerve are readily demonstrated by CT. CT is also the modality of choice in detecting and localizing most metallic and nonmetallic foreign bodies in relation to the globe, extraocular muscles, and optic nerve. The extent and size of subperiosteal hematomas may also be delineated with CT images.
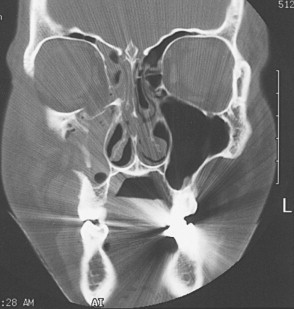
Patients with cervical spine or severe head injuries cannot be imaged in the direct coronal plane. In these patients, computer-reformatted coronal images can be obtained based on the axial image set. There is, however, loss of spatial resolution with computer-reformatted images. Also, the axial image set must be obtained with thin slices (1 to 3 mm). Small amounts of patient motion will introduce artifacts into the reformatted images. Sagittal or semisagittal reformatted images may occasionally show better delineation of anterior and posterior fracture margins than coronal or axial views.
CT imaging is not without limitations. Coronal CT is uncomfortable and often impossible to perform. Sedation is frequently required in pediatric patients. In these instances, the risk of the procedure versus the potential benefits must be weighed. The cost of CT scanning is higher than the cost of standard radiographs. Radiolucent foreign bodies such as wood are often missed on CT scan. In certain cases, echography and MRI are helpful in detecting radiolucent foreign bodies when CT scans are equivocal or negative.
Computer-generated three-dimensional CT images may provide improved depiction of complex orbital facial fractures ( Figure 13-13 ). They generate more spatial information and show fracture extent and fragment displacement in severe trauma with multiple fracture fragments. In these instances, three-dimensional CT can be a useful adjunct in surgical planning. Patients must remain absolutely motionless during the scan, which often requires over 100 axial slices to obtain adequate results. Thin fracture lines are, however, usually invisible on three-dimensional CT.
MRI has limited uses in orbital trauma and is less sensitive than CT in depicting fractures. MRI is useful for assessing soft tissue involvement, such as incarceration of extraocular muscles or orbital fat. It may be useful in evaluating orbital fractures if direct coronal CT is not obtainable or in cases of excessive dental artifact. Standard radiographs or CT scans should be obtained before MRI is performed on patients with suspected intraocular or intraorbital ferromagnetic bodies, because of the potential for displacement and significant ocular injury. Wood can appear isodense with fat and air on CT. This may result in misinterpretation of orbital scans. MRI has been used to identify wood and foreign bodies that have been misdiagnosed by CT scan. MRI should be considered when penetration of wooden foreign bodies is likely and when an apparent air collection within the post-traumatic orbit does not rapidly resorb, which suggests a space-occupying foreign body.
A correlation between ocular motility and evaluation of computed tomography in orbital blow-out fractures was retrospectively studied in 113 cases of pure blow-out fracture with diplopia. Patient’s satisfaction with treatment was based on percentage of Hess area ratio (HAR%) on the Hess chart. Fracture type and the number of points of contact of extraocular muscles to the fracture edge were recorded from CT findings. Initial CT findings of the rectus muscle’s contact with the fracture edge were strongly correlated with surgical outcome.
Postoperative ocular motility and residual diplopia were retrospectively studied in 30 patients with orbital blow-out fractures by correlating preoperative CT findings. The authors concluded that when the CT-depicted relationship between bone fragments and soft tissue is considered, greater degrees of soft tissue incarceration or displacement appear to result in poorer motility outcomes. An urgent surgical approach to selected orbital injuries was recommended.
Ophthalmic echography may be used occasionally to evaluate traumatic injuries of the orbit. It is a readily available, safe, inexpensive, noninvasive imaging modality. Foreign bodies located in the anterior orbit may be identified with ultrasonography; however, because of highly reflective orbital structures such as fat and bone, the foreign-body signal may be obscured when the object is located in the orbital apex. Wood and other radiolucent material that may not be visible on CT scan can be detected with ultrasonography. Color Doppler imaging (CDI) is a more recent ultrasound technique that gives simultaneous two-dimensional imaging of structure and blood flow. CDI is useful in evaluating post-traumatic high-flow carotid cavernous fistulae. However, angiography remains the definitive procedure in establishing the diagnosis.
ASSOCIATED INJURIES
Patients sustaining midfacial trauma often have concomitant neurologic or multisystem injuries, which can make proper orbital evaluation difficult. An unconscious or uncooperative patient poses additional challenges. Only after the primary trauma survey and initial patient stabilization have been performed can attention be directed toward suspected orbital trauma.
Bony orbital trauma with wall fracture can result from either blunt or penetrating trauma. An obvious and significant blunt force injury can mask a more subtle concomitant penetrating injury, particularly in a motor vehicle accident. Other injuries associated with orbital fractures are as follows:
- •
Midfacial fractures such as zygomatic complex fractures, NOE fractures, frontal sinus fractures, and LeFort II and III fractures involving the facial skeleton. These are often associated with more severe injuries of the orbit and globe.
- •
Basilar skull fractures, corresponding to the mechanism by which they are sustained. These fractures are usually caused by high-velocity impact and are often accompanied by serious neurologic injuries.
- •
Superior orbital fissure syndrome or orbital apex syndrome. The former consists of dysfunction of cranial nerves III, IV, V, and VI because of compression by fractured bony segments or hematoma. The latter consists of superior orbital fissure syndrome with optic nerve injury ( Figure 13-14 ).
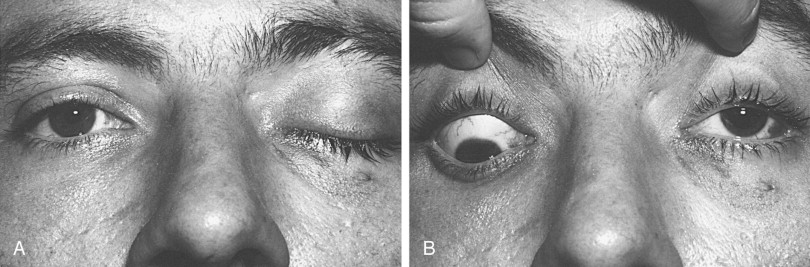
FIGURE 13-14 Superior orbital fissure syndrome of the left eye. A, Ptosis of left upper lid as a result of cranial nerve III involvement. B, With the examiner retracting the upper lids and the patient in downward gaze, the left eye does not deviate from the central axis (primary gaze). This is due to involvement of cranial nerves III and IV; cranial nerves V and VI would also be involved. The optic nerve was not involved. - •
Injuries to the globe that result in visual impairment. Between 0.6% and 4% of patients with orbital fractures are reported to suffer significant or total loss of vision in one eye.
OPHTHALMIC ASPECTS
VISUAL DISTURBANCES
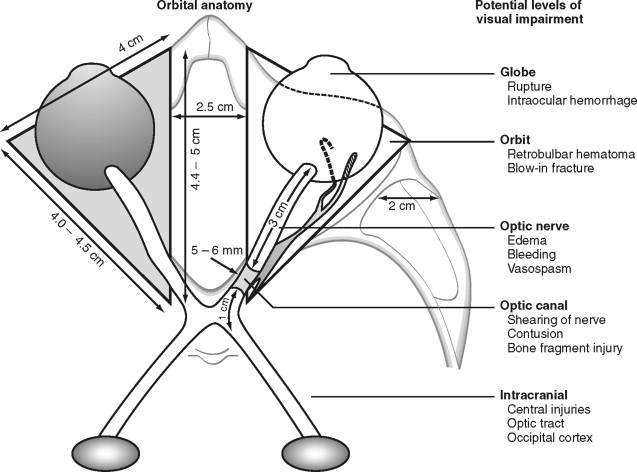
Visual impairment or loss can occur at various levels of the optic pathway, such as the globe or optic nerve (see Figure 13-2 ). Direct injury or forces transmitted to the globe can result in retrobulbar hematoma formation, rupture of the globe, hyphema, lens displacement, vitreous hemorrhage, and retinal detachment.
Retrobulbar hematoma is often manifested as visual loss with severe ocular pain. It causes an elevation in the intraorbital pressure, leading to central retinal artery compression or ischemia of the optic nerve ( Figure 13-15 ). Increased intraorbital pressure may also raise intraocular pressure, thereby compromising ocular circulation. Immediate surgical management is necessary and consists of a lateral canthotomy (with or without cantholysis) and blunt dissection along the lateral orbital walls to evacuate the hematoma. Often a drain is left in place for 24 to 48 hours to ensure that the bleeding has access to the surface. If necessary, intravenous mannitol and acetazolamide, along with a topical beta blocker, can be administered to lower intraocular pressure. In our experience, orbital volume reduction as a result of blow-in or compressive wall fractures does not typically cause increased intraorbital pressures leading to visual loss, because the fractures are often accompanied by some degree of “compensatory disruption” of the medial wall (ethmoid air cells) and floor (maxillary sinus). Through these drainage pathways and volume expansion, some degree of protection is provided against critical increases in intraorbital pressures.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses