Midfacial trauma commonly causes ocular injuries of varying degrees. Injury to the eye occurs in the majority of patients who have sustained midfacial trauma severe enough to cause a fracture, and approximately 15% have decreased vision. Isolated orbital blow-out fractures will have an associated eye injury in up to one third of patients. Both prospective and retrospective studies of patients who have sustained midfacial fractures indicate that as many as 20% may sustain serious ocular injury that warrants ophthalmologic referral.
The globe is protected from injury by the prominence of the orbital bones, but the anterior portion is less protected. An estimated 1.9 million reported eye injury cases were treated during the year 2001 in the United States. Orbital fractures with or without globe injury may result from varying degrees of facial trauma, and seemingly minor trauma may cause a fracture. The supraorbital and lower maxillary regions are more resistant to trauma when compared to the glabella area, the nasal bridge, and the malar eminence. In contrast, the resilient structure of the globe allows it to withstand blows of considerable force without rupture.
Orbital floor fractures have been associated with a 33% incidence of ophthalmic complications. The injuries may range in severity from a mild corneal abrasion and traumatic iritis to optic nerve avulsion and globe rupture. Some ophthalmic injuries are immediately obvious, but other less overt complications must be excluded. Examination of the eyes is mandatory for every patient who has sustained midfacial trauma severe enough to cause a fracture since inadequate care can result in blindness.
This chapter reviews the ophthalmic examination, discusses ophthalmic consequences of facial injury, and provides guidelines for referral to an ophthalmologist.
OPHTHALMIC ASSESSMENT
The assessment is composed of the history, assessment of vision, and examination of the eye and adnexal tissues.
HISTORY
Vision before the injury is important information, and a record of acuity exists if the patient has an optometrist or ophthalmologist. The following information should be sought but may not be attainable because of the severity of the injury:
- 1.
The time, place, and circumstances of the injury, including any loss of consciousness.
- 2.
The exact nature of the injury, and any possibility of intraocular or intraorbital foreign body.
- 3.
The type of the object that caused the injury (objects with a diameter greater than that of the orbit, such as fists, baseballs, and tennis balls, are more likely to cause blow-out orbital fracture than a small ball that hits the globe and causes globe perforation without fracture).
- 4.
The amount, duration, and direction of the traumatic force.
- 5.
Occurrence of diplopia, epistaxis, or rhinorrhea.
- 6.
Whether eyeglasses were worn at the time of injury, as they may have protected the eye or caused a glass foreign body.
- 7.
Past ocular history involving visual status, prior trauma, intraocular surgery, strabismus, and amblyopia.
CLINICAL EXAMINATION
Assessment of Visual Function
Visual acuity (VA) measures the resolving power of the eye and is determined in cases of midfacial trauma and facial fractures when possible. In optimal conditions the distance acuity is recorded as a fraction and is assessed with the patient at 6 meters (20 feet) from a Snellen chart. Each letter subtends 5 minutes of arc at a specific distance represented by the denominator. The numerator denotes the distance at which the VA is being tested (20 feet). For example, with a 20/40 VA the letters subtend an angle of 5 minutes at 40 feet or subtend 10 minutes of arc when viewed at 20 feet. The letters in a 20/20 line subtend 5 minutes of arc at 20 feet and a patient with “normal” vision should be able to resolve this size character.
One eye must be fully covered while acuity is determined. If the patient cannot read at 20/20 and yet does not have glasses for distance, acuity may be measured with the patient looking through a pinhole ( Figure 7-1 ). The pinhole corrects for large refractive errors by eliminating errant rays caused by refractive errors and allowing only the central straight rays of light to reach the retina. These straight rays are in focus on the retina and legible to the patient. Thus improvement in visual acuity using a pinhole means a refractive error is likely present.
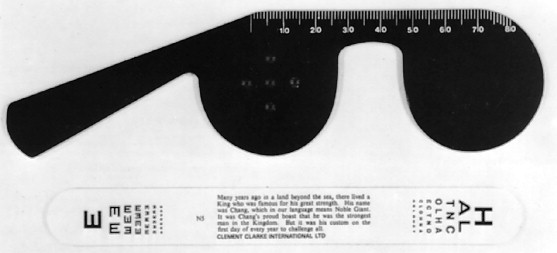
When visual acuity is less than 20/200, the patient is moved closer to the chart and the distance at which the top letter can be read is recorded (e.g., 10/200). If the chart cannot be read, the patient is asked to count fingers (CF) at a near distance, and this distance is recorded. If acuity is worse than this, hand movement (HM) perception or light perception (LP) is recorded.
In some patients with multiple injuries, it may be possible to assess only the visual acuity for near vision. The reduced Snellen letters at 0.33 m directly correlate with the full Snellen letters at 6 m. The near vision is recorded as Jaeger values, where J1+ is equivalent to Snellen 20/20 and J14 to 20/200. Pocket vision cards using the Jaeger system are readily available and easy to use. For older patients, near acuity must be determined with the use of reading glasses or a pinhole because the eye’s ability to accommodate on a near target declines with age. The physician may also use convex lenses (+2.00 or +3.00) in the absence of reading glasses. If a formal means of visual acuity assessment is not available, the clinician can estimate visual acuity using small print or can obtain a subjective vision report from the patient by asking if the patient’s vision is the same or worse than before the injury.
Testing Central Visual Function
Central visual function can be assessed by multiple methods other than visual acuity. One such method is color desaturation in which the patient is instructed to look at a red object with each eye in turn and compare the color. Color desaturation, or decreased intensity of the color, is present with an optic neuropathy. Light intensity is another subjective test in which the patient looks at a light source with each eye and compares the intensity of light between the eyes. This is recorded as a percentage and the intensity is decreased in the injured eye. Another easy test is to have the patient look at the examiner’s nose with each eye in turn and then have the patient determine if any part of the examiner’s face appears to be missing. This test may determine if small blind spots from an injury are present.
Visual Acuity in Children
The adult Snellen chart (naming letters or numbers) is preferred whenever possible but the most practical method for a child depends on age and cooperation. From the ages of 3 to 5, symbol naming or matching with tumbling E’s is preferred. For symbol matching, the child matches a symbol that is 20 feet away with an identical one on a chart held close to the child. In younger children, tests such as preferential looking, visual evoked potential (VEP), and optokinetic nystagmus (OKN) are helpful in grading the acuity, but these tests are less practical in a trauma situation. In infants, a central, steady, and maintained look following a light source is considered as a positive measure of acuity.
Visual Fields
Visual fields, at least with confrontation, are assessed in all patients with trauma. Confrontation visual fields are used to screen for visual field defects and a more detailed exam is undertaken for patients who have sustained a severe head trauma or have a defect in their vision. Automated visual field testing is a sensitive method in determining visual field defects and delineating the full extent of such defects. It is important to ask about any history of eye diseases, such as age-related macular degeneration or glaucoma, that could have already impacted the visual fields.
Assessment of the Central and Peripheral Visual Fields.
Traumatic damage to the visual pathways is more likely to cause impairment of the central 30 degrees of the visual field than of the periphery. Defects can be identified by both automated and manual perimetry, and attention can be placed on either the central or the peripheral fields. Automated perimetry can be performed to accurately determine the pattern and extent of the defect using a computer program. The patient looks into a perimeter (a semicircular bowl with flashing lights) and clicks a button to indicate that he or she sees the lights presented. Smaller, dimmer targets are presented in the central visual field because the macula is more sensitive, and larger, brighter lights are presented in the peripheral visual fields. Alternatively, tangent screen visual field testing can be performed by having a tester present point stimuli on the tip of a wand against a screen, manually plotting the visual fields.
The Pupils
The pupils should be examined in all cases of periocular trauma, and they can provide valuable information in the unconscious patient. If the patient’s visual acuity is reduced and shows no improvement with use of the pinhole, the pupils are tested for an afferent pupillary defect. This will be present in optic nerve damage anterior to the chiasm, retinal detachments, and extensive retinal damage. The pupil size should be measured in light and dark, and any anisometropia (pupil asymmetry) should be noted.
Direct and Consensual Pupillary Reflexes.
Pupils should be evaluated without significant background light to allow pupil dilation, and the patient relaxes his or her accommodation by fixating into the distance. A light source is used to illuminate the eyes from below, but not from the front because such lighting could cause an accommodative reflex. The light is shined into one eye and the direct response is noted. The light is shined into the same eye and the response of the contralateral pupil is observed (consensual reflex). The procedure is repeated for the other eye. Large afferent pupillary defects can be detected in this manner because the pupil will react poorly to direct stimulation but briskly to consensual stimulation.
Swinging Flashlight Test.
This test is used to detect more subtle relative afferent pupillary defects, such as those found when the optic nerve has been partially damaged. The term “relative” is used because this test compares the two optic nerves, and bilateral optic nerve damage of equal magnitude will not produce an abnormal test result. The pupils are illuminated in the same way, but the light is shined into each eye for about 2 seconds and then swung rapidly to the other eye. The eye with nerve damage will dilate slightly upon illumination and the normal eye will constrict slightly with illumination. For example, when a left incomplete or relative afferent pupillary defect is present, illumination of the left eye results in constriction of both pupils. When the light is swung to the right eye, both pupils constrict further. Re-illumination of the left eye results in slight pupillary dilation of both pupils until they return to their previous resting position.
This method can be used even in the presence of a unilateral dilated or minimally reactive pupil, which may occur with trauma, palsy of cranial nerve III, or pharmacologic dilation. The size of the reactive pupil is determined for both its direct and its consensual reflexes during the swinging flashlight test. A relative afferent pupillary defect is present if there is any change in the size of the pupil. (When the light is swung to illuminate the reactive pupil, an afferent defect is present on that side if the pupil dilates slightly. If the reactive pupil constricts upon illumination, the nerve defect is present on the contralateral side.)
EXAMINATION FOR STRUCTURAL DISORDERS
Periocular trauma can involve the bony structures, surrounding soft tissues, and the globe. Careful inspection will give the examiner several clues about underlying conditions that may be present.
Examination of the Anterior Segment
The anterior segment of the eye needs to be examined with magnification and an external light source, preferably with a slit-lamp biomicroscope, or alternatively with operating loupes. The anterior segment of the eye—involving the lids and adnexa, conjunctiva, cornea, anterior chamber, iris, and lens—is examined carefully for any pathologic conditions, including lacerations, abrasions, hemorrhage, perforations, foreign body, or lens dislocation, described later in this chapter.
Examination of the Posterior Segment
A thorough fundus examination with the aid of a magnifying lens and slit-lamp biomicroscope or an indirect ophthalmoscope is essential for posterior segment evaluation. This requires ophthalmic consultation as these skills take years of ophthalmic practice to master. This examination provides information about vitreous hemorrhage, retinal pathology, choroidal rupture, globe integrity, and optic nerve damage. It is very important to examine both eyes, instead of only focusing on the injured eye.
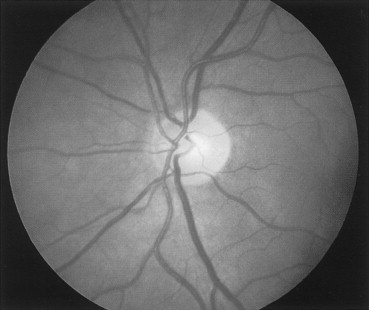
The direct ophthalmoscope is readily available and is a good tool when evaluating the posterior segment. It is easier to use and can be substituted when a slit-lamp or a magnifying lens is not available. The ophthalmoscope will also be helpful to identify any opacities inside the eye, such as vitreous hemorrhage or traumatic cataract. The normal appearance of the retina is shown in Figure 7-2 .
Dilated Examination of the Eye.
The dilated funduscopic examination should be performed in all patients with reduced visual acuity. Although the optic disc and macular region can be seen through an undilated pupil, the surrounding retina cannot be visualized well. Tropicamide 1% and/or cyclopentolate 1% dilate the pupil rapidly in patients with lighter colored irises, and the effect lasts only 4 hours while having minimal effect on accommodation. Adding phenylephrine 2.5% or 10% in patients with darker irises can help with dilation but caution must be taken in patients with cardiac dysrhythmias or hypertension or those taking monoamine oxidase inhibitors. On average, the pupil will dilate within 20 minutes. Not all patients can have their pupils dilated and the contraindications are as follows:
- 1.
A shallow anterior chamber; this may lead to angle-closure glaucoma.
- 2.
A history of intermittent blurring of vision, halos, and pain in the eye, suggestive of angle-closure glaucoma.
- 3.
An iris-supported intraocular lens, which could be dislodged if the pupil was dilated.
- 4.
Frequent neurologic exams in patients with intracranial injury that include monitoring the pupils.
Ophthalmic consultation may be necessary if there is any doubt about potential angle-closure or structural anomalies within the anterior chamber that would preclude dilation.
Once the pupil has been dilated, the posterior segment of the eye is easier to examine. The red reflex of the fundus can be seen by looking at the patient through the direct ophthalmoscope while standing at arm’s length. Darkening of this reflex may indicate that intraocular pathology is present. For the practitioner to view the fovea, the patient is asked to look directly at the light. Next, the patient is asked to move his or her eyes sequentially for 360 degrees so the entire retina can be seen. When the patient looks down, the examiner is looking at the lower half of the retina. The same is true when the patient looks in other directions.
Examination of Extraocular Motility
Facial and head injuries often lead to impairment of extraocular motility, especially in those with orbital fractures. The primary actions of each of the extraocular muscles need to be assessed and are shown in Figure 7-3 . Motility patterns can give clues to the site of injury and this will be explained later in the chapter. New-onset double vision indicates that a motility disorder is present and this must be carefully investigated. When the patient has an obvious ocular deviation without double vision, this means either that the patient had strabismus before the injury or that the vision in one eye is extremely poor. Past ocular history can help differentiate these causes.
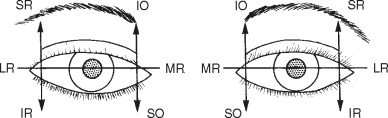
Examining ocular motility should be performed slowly and thoroughly, and a point source of light can be a useful tool for this exam by allowing the patient to perceive more subtle double vision. The patient should be instructed to state when double vision becomes manifest and the examiner should look at each eye for a deviation. Smaller deviations can be missed easily and the responses from cooperative patients provide valuable information. When the patient cannot or does not report double vision, each eye should be examined for deviations in all fields of gaze. A difference in the position of the corneal light reflex (Hirschberg’s reflex) may help as well, especially in uncooperative patients or children.
Another practical method in evaluation of diplopia is the red-glass test. A red plane glass is placed in front of one eye, and the light source is moved in positions of gaze as described. The red glass helps dissociate the eyes, and makes diplopia more recognizable for the patient. They will perceive both a white light and a red light when diplopia is present.
The cover-uncover test can also be used to determine when a deviation is present. The examiner sits in front of the patient and has the patient fixate on an object across the room. A card is placed in front of one eye while the examiner watches the uncovered eye. The eye will not move if it is fixated on the object. If the eye moves, the uncovered eye was not aligned with the covered eye and a deviation is present. Both eyes need to be checked in turn to be sure the eyes are aligned. This test can be performed in all fields of gaze to determine where the deviation is present.
Forced Duction Test.
Forced duction testing is a valuable test of the extraocular muscles when eye motility is limited. This can help differentiate between an incarcerated muscle and a paretic muscle. For example, when a patient has difficulty looking upward, either the inferior rectus could be entrapped or the superior rectus could be paretic. The test is performed by instilling a topical anesthetic (tetracaine or proparacaine) onto the surface of both eyes. A cotton-tipped applicator soaked in anesthetic and held on the conjunctiva for 1 minute provides maximal comfort. A small forceps is used to grasp the conjunctiva and extraocular muscle insertion about 8 mm from the cornea, and the globe is rotated both toward and away from the muscle. The contralateral eye is used as a control. If resistance is encountered when the globe is rotated away from the action of the muscle, entrapment may be present. A lack of resistance implies that paresis of the antagonistic muscle exists. (In a patient with an elevation deficit of one eye, either the inferior rectus is entrapped or the superior rectus is paretic. The examiner grasps the conjunctiva over the insertion of the inferior rectus muscle and rotates the globe upward and downward. Resistance with upward rotation means the muscle is entrapped, and no resistance means the superior rectus is paretic.)
Forced duction testing is also performed preoperatively in patients with entrapped muscles undergoing repair, and repeated after the repair to determine the operative success of releasing the muscle. The contralateral eye is again used as a control.
Examination of the Orbit
Examination of the orbit should include the bony structures, globe position, and the surrounding soft tissues. (Soft tissue injuries are discussed later in this chapter.) Edema, ecchymosis, and crepitus may indicate that the orbit has sustained serious injuries such as fractures or hematomas. Globe position and intercanthal distance should be reported as well because abnormalities with these indicate that severe injury is present. Bony deformities can often be palpated but this can be masked by extensive amounts of soft tissue edema. Foreign bodies must be sought when there is disruption of the soft tissues and clinical suspicion may be heightened based upon the mechanism of trauma.
The position of the globe may indicate where the pathology is present (e.g., lateral globe displacement from a process in the medial orbit). Also a widened intercanthal distance may exist in naso-orbital ethmoidal fractures. Soft tissue wounds are potential entry sites for foreign bodies and need thorough examination. Clinical findings assist the evaluation of radiographic imaging such as computed tomography (CT), which can help determine the full extent of injuries and the location of foreign bodies.
Anteroposterior Globe Displacement (Enophthalmos or Exophthalmos).
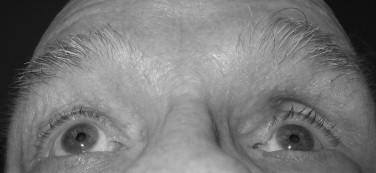
A number of traumatic mechanisms can cause anterior or posterior positioning of the globe. Immediately following injury, anterior protrusion of the globe, or exophthalmos, may be present as a result of soft tissue edema, hemorrhage, and bony fragments within the orbit. As the soft tissue swelling diminishes, posterior positioning of the globe, or enophthalmos, may develop. Alternatively, enophthalmos may be seen immediately following injury when a large orbital floor defect is present. Inspection may reveal a deepening of the supratarsal sulcus and pseudoptosis of the upper eyelid secondary to backward disposition of the globe.
The anteroposterior displacement is most accurately measured with an exophthalmometer by comparing the globe position in relation to the other globe, and a difference of more than 2 mm is considered abnormal. This instrument, however, uses the lateral orbital margin as the reference point and cannot be used in cases in which the orbital rim has been displaced. Another means of assessment in these cases is to position the back of the patient’s chair at a 45-degree angle or to ask the patient to lift his/her chin up, and then examine the patient from below, comparing the positions of the corneas with respect to the malar surfaces. A patient with leftsided enophthalmos is shown in the chin-up position in Figure 7-4 .
NONPERFORATING EYE INJURIES
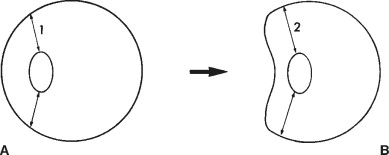
Blunt trauma to the eye can cause both direct and compressive injuries. Direct injury results from concussive forces striking the eye. Compressive injuries occur when a force presses against the globe, temporarily shortening its anteroposterior dimension and lengthening the vertical dimensions ( Figure 7-5 ). The internal structures of the eye that are circumferentially attached may tear from their insertions as a result. The various anatomic locations and associated nonperforating injuries are described.
CONJUNCTIVA
Trauma to the surface of the eye often leads to subconjunctival hemorrhage. This appears as painless, bright red blood on the surface of the eye beneath the conjunctiva and may be associated with chemosis, or edema. Bleeding may be localized from a vessel within the conjunctiva, or in deeper injuries it may extend from the orbit and track anteriorly. Orbital injury will have associated signs such as exophthalmos, limitation of ocular motility, and periocular ecchymosis. Patients with 360 degrees of subconjunctival hemorrhage and diminished vision may require surgical exploration to rule out an occult rupture of the eye. Lacerations of the conjunctiva need to be completely explored to ensure that deeper injury does not exist, and larger lacerations (>10 mm) should be sutured.
CORNEA
Abrasion of the corneal surface with or without a foreign body causes severe pain, blurry vision, tearing, and photophobia. An abrasion results when the epithelial surface of the eye is disrupted for any reason ( Figure 7-6 ). Direct trauma, prolonged exposure to air, or contact with caustic agents (e.g., cleaning agents, alcohol) all can cause an abrasion. The diagnosis is made by fluorescein staining of the denuded epithelium, which will fluoresce with blue light. A topical anesthetic will provide enough relief to permit examination.
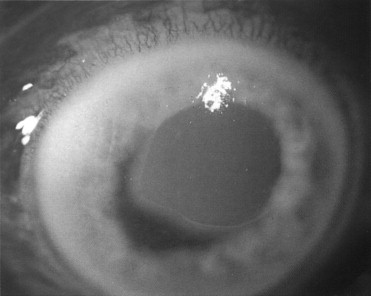
Slit-lamp biomicroscopy is recommended to determine the full extent of any eye surface injury. When the patient has been struck by a piece of metal with high velocity (e.g., hammering metal on metal), foreign bodies and penetration of the globe should be suspected. The depth of the wound needs to be clearly visualized since small penetrating injuries can be self-sealing.
Abrasions leave the corneal stroma vulnerable to infection and are treated with topical antibiotics until the protective epithelium can regenerate. Small abrasions often heal within 24 hours and these patients need daily examinations until the abrasion is gone. Larger abrasions with associated photophobia can be treated with an additional cycloplegic drop, such as cyclopentolate. This treats the pain of ciliary body spasm by temporarily paralyzing the muscle. A topical nonsteroidal anti-inflammatory drug may also be of benefit.
If a foreign body is present, it should be removed with great care using binocular magnification if possible. A hypodermic needle held tangential to the corneal surface is used to elevate the foreign body after a topical anesthetic has been instilled. The patient must not move, and the forehead band on the slit-lamp prevents the patient from moving forward towards the needle. The patient is encouraged to rest with both eyes closed in order to prevent the continuous rubbing action of the eyelids over the fragile healing epithelium. The patient is examined daily until the epithelium has healed, and topical antibiotics are necessary on a frequent basis.
Blunt injury can also cause edema of the cornea by affecting the innermost corneal layer composed of endothelial cells. Damage to the endothelium may result from direct contusion, increased intraocular pressure, and/or reactive inflammation. This monolayer of cells continuously pumps water out of the corneal stroma, providing the clarity necessary for vision. Damage to these cells produces an opaque, thickened, edematous cornea. The edema usually resolves spontaneously but may require topical hyperosmotic medication and/or corneal grafting.
ANTERIOR CHAMBER
The anatomic space between the cornea and the iris is the anterior chamber, which is filled with aqueous fluid secreted from the ciliary processes. Eye trauma can tear iris and ciliary body blood vessels, resulting in bleeding in the anterior chamber and inflammation. Also, the anterior chamber may become deep or shallow depending on the extent and location of the injury.
Hyphema
A hyphema is blood in the anterior chamber and most often results from tearing of blood vessels at the root of the iris ( Figure 7-7 ). The patient usually presents with pain, photophobia, and blurred vision, and red blood cells can be seen in the anterior chamber. Microscopic evidence of red blood cells in the aqueous without layering inferiorly is called a microhyphema. Larger amounts of blood can be seen without magnification and will collect along the bottom of the chamber when the patient is upright. The prognosis is related to the amount of blood present. The height of the blood needs to be measured daily and this can be followed as a clinical sign of improvement or worsening.
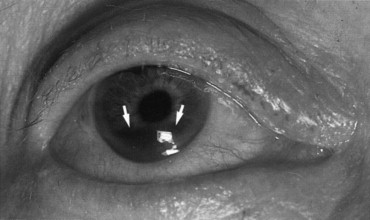
Patients are prescribed bed rest to minimize rebleeding, and the head of the bed should be elevated at least 45 degrees at all times. Atropine 1% decreases the pain and helps constrict the blood vessels, and prednisolone 1% decreases intraocular inflammation. Aminocaproic acid may be used in the early course of treatment for larger hyphemas. The intraocular pressure must be evaluated daily because blood and inflammation may impair aqueous outflow. In the event of raised intraocular pressure, corneal blood staining may result, necessitating washout of the anterior chamber on an urgent basis. Other indications for surgery are significant visual deterioration, total filling of blood in the anterior chamber, persistent clot in the angle for 7 days, and increased intraocular pressure despite medical treatment (>50 mm Hg for 5 days or >35 mm Hg for 7 days).
In most cases, spontaneous resorption of hemorrhage takes place, but in a small proportion of cases the eye may rebleed. Rebleeding commonly occurs around the fourth day, when clot remodeling and contraction occurs. Aspirin and nonsteroidal anti-inflammatory medications increase the incidence of rebleeding and are contraindicated. Also, children carry a risk of amblyopia, and therefore should be followed and treated more aggressively. Finally, patients of Mediterranean and African descent should be questioned about the possibility of sickle cell disease since they are at higher risk of more serious complications from the hyphema.
Abnormal Depth of the Anterior Chamber
An abnormal depth of the anterior chamber is a sign of damage to the eye, and either a shallow or a deep chamber provides clues to the site of pathology. The distance between the cornea and the iris can be evaluated by observing the eye from an oblique angle, and this is performed most effectively using an external source of light and high magnification. A shallow anterior chamber can result from low pressure within the eye, angle-closure glaucoma, or posterior pressure from blood or edema within the posterior segment of the eye. A deep anterior chamber may indicate that the iris and/or lens is torn from its insertion, or the eye could be ruptured. Intraocular pressure helps differentiate these pathologic processes.
IRIS AND PUPIL
Traumatic Iritis
Traumatic iritis usually develops 2 to 3 days after injury to the eye and is manifest by pain, photophobia, tearing, and redness. When the iris is traumatized, it becomes inflamed, allowing leakage of proteins into the anterior chamber. The inflammatory white blood cells and protein (cells and flare) can be visualized and quantified, providing objective measurements to assess iritis severity and response to therapy. Treatment for traumatic iritis includes topical steroids (e.g., dexamethasone or prednisolone) and a short-acting pupillary dilator (cyclopentolate) that will help prevent the inflamed iris from adhering to the lens capsule and creating posterior synechiae.
Blunt eye trauma can tear the pupillary sphincter, leading to dilation of the pupil (traumatic mydriasis). This damage is permanent and the pupil may react poorly or not at all depending on the extent of injury. On slit-lamp examination, the pupil remains dilated and tears can be seen within the pupil margin and stroma ( Figure 7-8 ). In cases in which there is no obvious damage to the iris, one must consider other causes of a dilated pupil such as a third cranial nerve palsy. Occasionally when the iris sustains trauma, a pigment ring from the pupillary border is deposited on the center of the lens capsule (Vossius’ ring). This sign indicates that the eye has been severely injured and should be examined thoroughly.
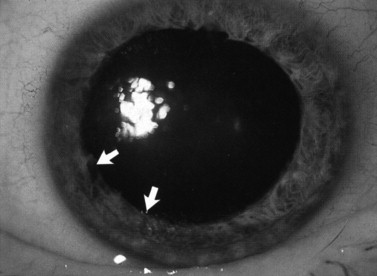
A partial tear of the iris at its insertion leads to angle recession, which can damage the drainage system of the eye. Aqueous fluid drains through the trabecular meshwork located in the angle, and disruption of this structure may cause the intraocular pressure to increase. All trauma to the iris may cause glaucoma, which will be expounded upon later in this chapter. Deepening of the anterior chamber with angle recession is less obvious and ophthalmic consultation is necessary. The diagnosis is made using a gonioscopic lens to see a widening of the angle of the anterior chamber.
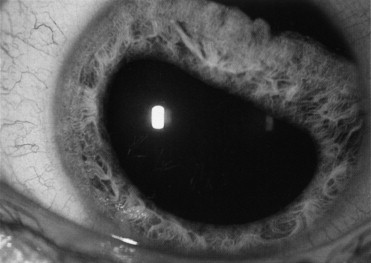
More severe damage to the iris can cause an iridodialysis, which is a tearing of the iris from its root ( Figure 7-9 ). The damage can extend up to 360 degrees within the eye and the full extent needs to be noted. Using direct ophthalmoscopy and the slit-lamp, a gap can be seen near the corneal limbus where the iris used to insert. Blood and pigment particles are usually present in the anterior chamber from torn blood vessels and the torn iris.
POST-TRAUMATIC INTRAOCULAR PRESSURE CHANGE
Hypotony is defined as an abnormally low intraocular pressure—less than 6 mm Hg. It is associated with open globe injury, choroidal detachment, retinal detachment, ciliary body shut-down, and cyclodialysis cleft (disinsertion of the ciliary body from the sclera at the scleral spur). A persistent decrease in intraocular pressure can lead to decreased visual function with hypotony maculopathy, suprachoroidal hemorrhage, retinal vascular tortuosity, and chorioretinal folds with image distortion.
Post-traumatic glaucoma is a triad of increased intraocular pressure (IOP) above 22 mm Hg, increased optic cup to disc ratio (cupping), and visual field changes. Increased IOP following trauma occurs secondary to a variety of mechanisms. Acute-onset glaucoma without hyphema is often due to inflammatory cells from traumatic iritis causing blockage of the trabecular meshwork (TM). In cases of hyphema, increased IOP results from obstruction of the TM with red blood cells and debris. Ghost cell glaucoma occurs 2 to 3 weeks after penetrating or nonpenetrating injury that resulted in a vitreous hemorrhage. Red blood cells in the vitreous gel become rigid. They migrate into the anterior chamber and block the TM, leading to increased IOP.
Another mechanism for late-onset glaucoma is angle recession, that is, a tear in the ciliary body that splits the longitudinal from the circular muscle fibers. The incidence of glaucoma is related to the extent of recession. The incidence rises to 10% if more than 180 degrees of the angle is recessed. Angle recession leads to increased resistance to outflow from the TM and subsequent increase in IOP.
LENS
The lens is held in position by the zonule, which is composed of numerous fibers that are attached circumferentially to the ciliary body and the lens capsule. When the zonule fibers are partially torn, the lens loses support and subluxation of the lens may occur ( Figure 7-10 ). The patient may complain of blurry vision because the malpositioned lens is out of focus, or double vision may exist from light striking the edge of the lens. Slit-lamp examination when the eye is dilated shows excess movement of both the lens and the iris as the eye moves. The edge of the lens can be seen in the area where the zonule fibers have been torn. The angle should be examined using gonioscopy since the root of the iris has likely sustained injury.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


