INTRODUCTION
The embryologic events that initiate, control and guide formation of human odontogenic structures take place through an intricate and finely regulated series of inductive interactions between epithelium and mesenchyme, which begin during the fifth and sixth weeks of intrauterine life and are completed about the sixteenth year after birth. During this extended period, there is ample opportunity for developmental and growth mechanisms to fail in whole or in part, resulting in the formation of malformations, hamartomas , and neoplasms , collectively known as odontogenic tumors .
Odontogenic tumor, therefore, is an imprecise term that encompasses a wide spectrum of lesions and variants. Distinctions among these three forms of abnormalities may, in part, depend upon the embryologic stage of initiation and the histologic and gross appearance of the lesion at the age of clinical discovery. Overlap and combinations among lesions are possible and do occur. A malformation, although neoplastically innocent, because of size and/or anatomic position may cause a functional disturbance or aesthetic liability; a hamartoma by continued growth may eventuate as a neoplasm; and a benign odontogenic cyst or usually self-limiting neoplasm may give rise to an aggressive or malignant neoplasm. Thus, an odontogenic lesion may require surgical management because of physical presence or biologic behavior. Biologic behavior, biologic activity, and growth potential are three interrelated effectors that determine a lesion’s ability to harm the host.
Since histologic appearance and biologic behavior are not necessarily linked, assessment of the growth potential of an odontogenic lesion may be difficult. This is true particularly in the case of a “newly discovered” or rarely encountered entity of which long-term clinical experience has not been documented. Even in the instance of ameloblastoma, a lesion that has been recorded extensively for over a century, there are diametrically opposed management philosophies. In addition to so-called radical or conservative modalities, variations and combinations of treatment have been attempted.
The goal of treatment, of course, is eradication of a lesion with the least morbidity possible and the preservation or restoration of function. However, a common all-inclusive algorithm for the management of odontogenic tumors, as well as other maxillofacial jaw lesions, must await long-term treatment and outcome studies. The current basis for the decision whether, when, and how to manage a number of odontogenic lesions is contingent upon individual clinical factors and assessment of the lesions’ biologic behavior. Several odontogenic tumors have distinct histologic patterns, are well documented in case histories and case series studies, and have known outcome results; however, even among these lesions an occasional rogue lesion may exhibit unexpected biologic behavior.
Such activity may be regarded as chance vagary of the lesion but also may be due to unappreciated histologic or molecular variation with concomitant altered biologic behavior. Since histologic diversity may help to characterize odontogenic lesional behavior, it is prudent to consult an oral and maxillofacial pathologist for evaluation of odontogenic lesions. Presently most hospital pathology departments have an oral and maxillofacial pathologist on staff or routinely consult with them. This is to be expected and encouraged since odontogenic tumors comprise a specialized area of pathology.
CLASSIFICATIONS
It is interesting to read the earlier efforts at classification, especially by the British writers of the later nineteenth and early twentieth centuries, up to the 1930s as they labored to clarify and classify odontogenic lesions. The class name they adopted was Odontomes , under which the entire gamut of odontogenic lesions, as well as unrelated jaw lesions, were placed. In the United States, Ivy and Churchill also made an effort to bring order to and reclassify odontogenic lesions. In 1946 Thoma and Goldman published their classic paper, which described a classification of odontogenic tumors based on the tissue cell of origin and eliminated many extraneous lesions from consideration. They recognized lesions derived primarily from epithelium, those predominantly from mesenchyme, and a mixed group in which both epithelial and mesenchymal tissues shared in the formation of a lesion. Their scheme, and particularly the case illustrations, demonstrated the inductive effects of one tissue (epithelium) on another (mesenchyme) in the pathogenesis of odontogenic tumors.
Several other revisions and refinements were made by Pindborg and Clausen and by Gorlin and other colleagues, who also proposed a classification based on the embryologic inductive interactions of the tissues on each other. These classifications provided a better means to view and catalog lesions in an orderly manner but did not give the surgeon insight into their biologic behavior. In a further departure, the World Health Organization (WHO) published a classification in 1971, a revision in 1992, and a further revision in 2005 with input from international groups of oral and maxillofacial pathologists, which is based on embryologic induction patterns (1992) but also categorizes odontogenic lesions into benign and malignant types (1971, 1992, 2005). The authors recognize the inherent difficulties in this or any classification that contains lesions of uncertain status as well as lesions for which behavioral information is still being accumulated. As is always the case, the latest efforts at histologic typing of odontogenic tumors have evoked reasonable suggestions and comments from several writers. It is apparent that no classification is entirely satisfactory and that all have deficiencies. The order of presentation used in this chapter follows the traditional separation of lesions into epithelial, mesenchymal, and mixed types. In addition, an attempt is made to indicate a progression from the least to the most aggressive lesions. However, it may be emphasized again that known nonaggressive lesions may exhibit unexpected aggressive behavior.
CLASSIFICATIONS
It is interesting to read the earlier efforts at classification, especially by the British writers of the later nineteenth and early twentieth centuries, up to the 1930s as they labored to clarify and classify odontogenic lesions. The class name they adopted was Odontomes , under which the entire gamut of odontogenic lesions, as well as unrelated jaw lesions, were placed. In the United States, Ivy and Churchill also made an effort to bring order to and reclassify odontogenic lesions. In 1946 Thoma and Goldman published their classic paper, which described a classification of odontogenic tumors based on the tissue cell of origin and eliminated many extraneous lesions from consideration. They recognized lesions derived primarily from epithelium, those predominantly from mesenchyme, and a mixed group in which both epithelial and mesenchymal tissues shared in the formation of a lesion. Their scheme, and particularly the case illustrations, demonstrated the inductive effects of one tissue (epithelium) on another (mesenchyme) in the pathogenesis of odontogenic tumors.
Several other revisions and refinements were made by Pindborg and Clausen and by Gorlin and other colleagues, who also proposed a classification based on the embryologic inductive interactions of the tissues on each other. These classifications provided a better means to view and catalog lesions in an orderly manner but did not give the surgeon insight into their biologic behavior. In a further departure, the World Health Organization (WHO) published a classification in 1971, a revision in 1992, and a further revision in 2005 with input from international groups of oral and maxillofacial pathologists, which is based on embryologic induction patterns (1992) but also categorizes odontogenic lesions into benign and malignant types (1971, 1992, 2005). The authors recognize the inherent difficulties in this or any classification that contains lesions of uncertain status as well as lesions for which behavioral information is still being accumulated. As is always the case, the latest efforts at histologic typing of odontogenic tumors have evoked reasonable suggestions and comments from several writers. It is apparent that no classification is entirely satisfactory and that all have deficiencies. The order of presentation used in this chapter follows the traditional separation of lesions into epithelial, mesenchymal, and mixed types. In addition, an attempt is made to indicate a progression from the least to the most aggressive lesions. However, it may be emphasized again that known nonaggressive lesions may exhibit unexpected aggressive behavior.
GENERAL CLINICAL, DIAGNOSTIC, AND MANAGEMENT CONSIDERATIONS
CLINICAL
Odontogenic tumors, as a group, are relatively uncommon; several are rare, and a few are very rare. Published occurrence reports of odontogenic tumors, expressed as percentage of the total lesions in oral biopsy services of various institutions in the United States and countries throughout the world, reveal a low incidence. However, the percentage incidence of specific odontogenic tumors within the group appears to vary in different races and countries. The clinical importance of odontogenic tumors is not measured by their numbers, but because some lesions are very destructive and surgical management involves the face, oral tissues, and jaws of both young and old patients. Correlation of clinical, radiographic and histologic analyses is necessary to prevent over-or undertreatment.
There are no clinical signs, symptoms, or physical findings that permit diagnosis of specific odontogenic tumors. The age and sex of the patient and location characteristics of lesions are derived from pooled data compiled from the literature and unpublished cases. Such information is useful only when combined with other diagnostic features.
IMAGING
Since most odontogenic tumors emanate from or involve the jaws, imaging modalities, including plain radiography (periapical, occlusal, panoramic), computed tomography (CT), CT three-dimensional reconstruction, computer-generated models, magnetic resonance imaging (MRI) and positron emission tomography (PET) scan CT offer an unparalleled opportunity to view the tumor in relation to the jaws and adjacent soft tissues. Radiographic images do not provide pathognomonic identification of odontogenic lesions, since several odontogenic tumors as well as nonodontogenic lesions may share imaging characteristics. However, highly useful visual information can be gathered to study a lesion for presumptive differential diagnoses, selection of biopsy sites, and management decisions. A lesion’s position, size, and shape; presence or absence of lesional calcifications; estimation of soft tissue volume in relation to calcified tissue, cyst formation, and impingement on or inclusion of vital anatomic structures; displacement of teeth or root resorption; and interface boundaries between lesion and host bone help the clinician to form a characterization of the tumor’s activity and aggressiveness.
Odontogenic tumors are most often discovered by dental radiography (periapical, occlusal, panoramic) as part of routine screening during a dental visit, examination of a patient with a specific complaint (e.g., pain, oral soft tissue or facial enlargement), or elective consultation (e.g., orthodontic, orthognathic surgery). The judgment of the clinician will determine the extent and types of imaging that are needed or most useful for the management of a specific case. Yet, in many instances, there is a tendency to use fewer imaging modalities than are desirable, usually later regretted, since imaging provides a valuable documentary and study tool .
Overall viewing of bone and soft tissue by consecutive anatomic “slices” and planes is provided by CT and MRI respectively. The volume of the tumor and the interface margins between the tumor and surrounding bone and soft tissue is roughly revealed by PET scan-CT, obtained after injection of a glucose isotope conjugate metabolized by the tumor. The 3-D CT (three-dimensional computerized tomogram) permits viewing the lesion in almost kaleidoscopic anatomic displays; and the 3-D CT generated model provides an excellent physical reproduction of the anatomic part and tumor. The model is useful for planning or carrying out a mock surgical procedure and provides a mechanical frame upon which to contour a reconstruction appliance.
BIOPSY
Definitive diagnosis is established only after incisional, excisional, or intraoperative frozen section biopsy for histologic analysis. The specific biopsy technique is selected after careful assessment of the patient (age, physical health, emotional status) and of the use of local, sedation, or general anesthesia to ensure patient cooperation, gain access to the lesion, and obtain sufficient tissue for study. An incisional biopsy performed several weeks before definitive management often precludes excision or frozen section diagnostic “surprises.” Thus, time is gained to receive a soft or calcified tissue diagnosis, plan the surgical procedure, prepare and/or obtain special presurgical requirements, and fully discuss management with the patient or guardian. Excisional biopsy should be performed for completely calcified odontogenic lesions (i.e., malformations radiographically demonstrating only teeth or a cementum-like tissue) in which histologic diagnosis is not immediately essential, for physically impaired patients for whom several anesthetic or surgical exposures should be avoided, or for small lesions (approximately 1.0 to 1.5 cm in diameter) that can be excised completely and can reasonably be expected not to require further operation after histologic study. Intraoperative frozen section should be used to study questionable soft tissue encountered in areas not sampled by the incisional biopsy, and to examine the adequacy of the boundary between lesion and host bone and/or soft tissue in instances in which extensive resection is not planned. The preparation of a good frozen section is technique-sensitive and requires proper specimen orientation and avoidance of incorporated dense bone (which nicks the microtome blade and disrupts the tissue section).
The biopsy is an important procedure and must be planned and conducted carefully. Because of its key role in the diagnostic process, and later in the definitive surgical procedure, some aspects of the biopsy are discussed here in detail: (1) The lesion is approached, with radiographic guidance, by incision through intact crestal mucosa over intact bone, in order to preserve “noncontaminated” mucosal soft tissue that may be used for wound closure after a postbiopsy surgical procedure. (2) The lesion is aspirated through the intact bone to rule out a vascular lesion masquerading as an odontogenic tumor (other potential radiographic imitators of odontogenic tumors include aneurysmal bone cyst, giant cell lesion, and fibro-osseous disease). (3) Aspiration of cystic fluid neither confirms nor disproves the presence of a cystic odontogenic tumor; the fluid may or may not be retained for a cell-block preparation. (4) Entrance through the bone to the lesion should be selected and obtained without injury to nerves or teeth, if possible. (5) If the radiographs indicate variations in the lesion (solid, cystic, or calcified areas), a sufficient number of tissue samples should be removed for thorough histologic study, including tissue bordering the lesion-host bone interface. The specimens are incised for removal and not crushed with forceps or hemostats. (6) Before suture closure of the biopsy incision, if desirable, a nonresorbable tissue barrier can be placed over the bone entrance to the lesion to prevent fluid or lesional cell escape into noninvolved periosteum or mucosa.
The specimens are oriented and identified by marking the natural tissue surfaces with indelible ink of the same or a different color (i.e., to represent the biopsy sites such as anterior or posterior), spread flat with the tissue exudate side down on an absorbent surface (e.g., glove wrapper or filter paper), allowed to dry for about 3 to 4 minutes so that the specimen adheres to the carrier and does not curl, and placed into separate labeled containers. This technique permits the pathologist to identify and orient the frequently small tissue samples and prevents improper gross sectioning and poor histologic preparation.
The surgeon or assistant surgeon should complete the pathology laboratory form. This important function should not be assigned to others (e.g., circulating nurse), since accuracy cannot be assured later if questions concerning biopsy sites and histologic findings arise. The pathology laboratory form provides space for a brief history, clinical diagnosis, special instructions to the pathologist, and diagram of the lesion and lesional area to identify the orienting tissue ink marks. A radiograph (or good copy) of the lesion and anatomic site should accompany the specimen or be sent to the pathologist.
The histologic tissue slides should be reviewed by the surgeon alone or with the pathologist. Unusual, problematic, or unexpected findings should be discussed with the pathologist. After histologic study and diagnosis, the biologic activity of the lesion is estimated. An equivocal diagnosis or a choice among several diagnoses should initiate a review by other pathologists.
MANAGEMENT
The objectives of the surgical management of odontogenic tumors are the eradication of the lesion, preservation of normal tissue to the extent possible, and restoration of significant tissue loss, form, and function. All agree that the surgical procedure should be sufficient to the need; however, in many instances there is disagreement among surgeons and pathologists about the extent of surgery that specific tumors require. These differences of opinion are reflected in the literature and account for “conservative” or “radical” approaches to treatment that result from unanswered questions concerning the biologic activity of several odontogenic tumors.
Some surgeons maintain an almost mystical adherence to a conservative surgical approach, in the conviction that all odontogenic tumors are benign. This viewpoint amounts to a planned recurrence for some lesions. The basis for this opinion is the belief that a recurrence or recurrences (more properly, regrowth of residual lesion) can be managed by a simpler procedure that can avoid jaw resection. However, one of the results of such an approach is the creation of a shotgun pattern of recurrence by which the lesion is spread throughout a larger area, thus compounding the surgical problem. Other surgeons are routinely aggressive in their approach to all odontogenic tumors, and so sacrifice more normal tissue than is necessary to ablate the lesion. The argument has been made that a well-planned and executed resection and reconstruction serves the patient physically and emotionally better than repeated surgical procedures. The defense of or response to both positions lies in the continued accumulation and dissemination of accurate data and knowledge of odontogenic lesions. This can be achieved best by the surgeon and pathologist working together to correlate clinical, surgical, and histologic findings with biologic activity and clinical outcome. This effort would be aided greatly by the adoption of standardized, defined surgical excision terms acceptable to the entire surgical community.
One attempt to provide precisely defined excision terminology applicable to lesions in bone was offered by Gold and coworkers. All excisions involving bone can best be described by the following designations: enucleation, curettage, marsupialization, recontouring, resection without continuity defect (Rs–CD), resection with continuity defect (Rc–CD) and disarticulation. All are definable. Some may be used in combination, and three—Rs–CD, Rc–CD, and disarticulation—can be measured radiographically, grossly, and histologically. This terminology is employed in this chapter where germane. Certainly at present, there are no absolute standards of management for many odontogenic tumors. The treatment of several lesions continues to be debatable, and some suggestions for treatment made in this chapter may be in disagreement with the opinions of other writers.
EPITHELIAL ODONTOGENIC TUMORS
ADENOMATOID ODONTOGENIC TUMOR
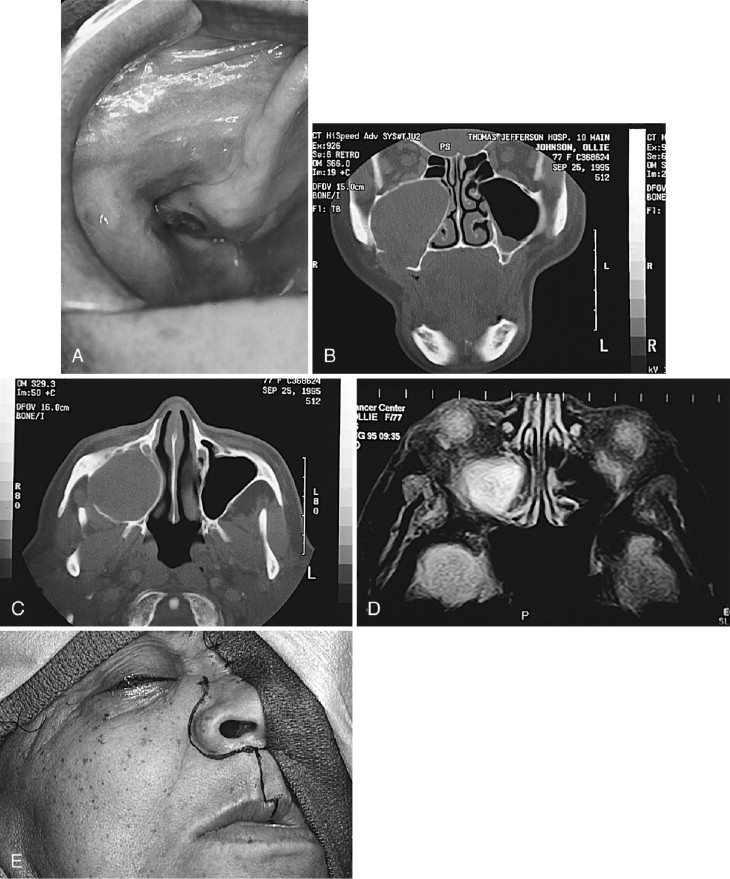
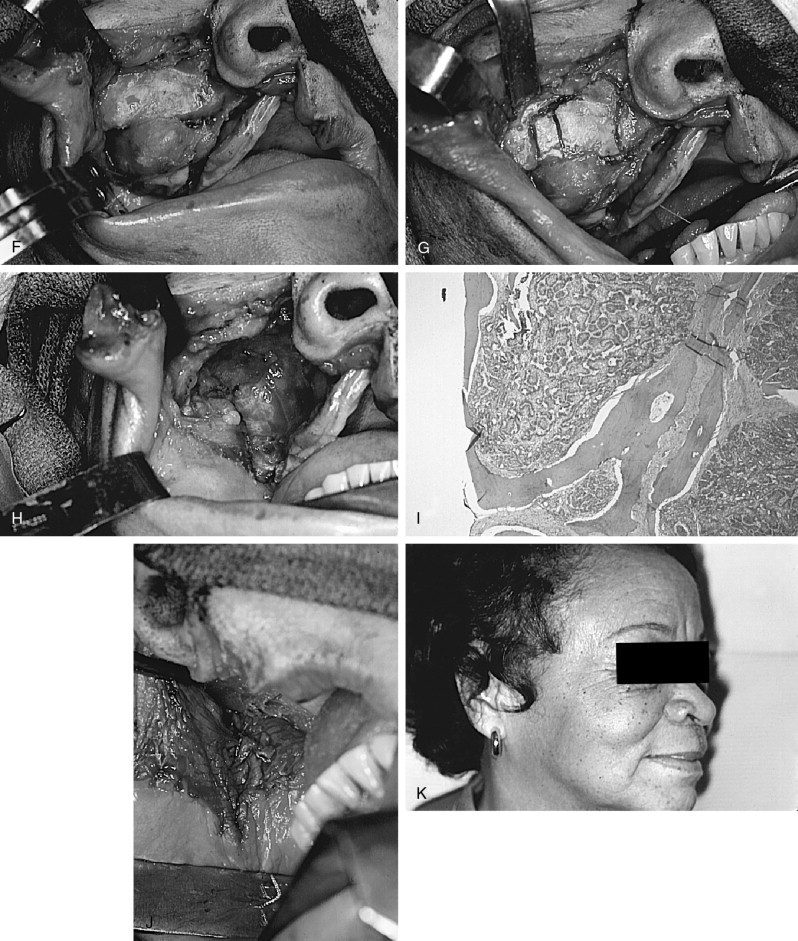
The adenomatoid odontogenic tumor (AOT) has been known by a number of descriptive names since it was first reported and later recognized as a distinct odontogenic lesion unrelated to ameloblastoma ( Figure 25-1 ). Although it was considered to be a variant of ameloblastoma at one time, surgical and treatment outcome experience has borne out the benign, nonaggressive nature of this lesion. Variously known as adenoameloblastoma, ameloblastic adenomatoid tumor, glandular ameloblastoma, and adenomatoid ameloblastoma, as well as by other designations, the lesion has been extensively recorded in the literature.
The acceptance of the more apt name adenomatoid odontogenic tumor recognizes the unique and peculiar ductlike or tubelike histologic structures and odontogenic origin but removes the burden of ameloblastic stigmatization. The AOT can be considered a hamartoma with occasional neoplastic behavior .
Clinicopathologic Considerations
The adenomatoid odontogenic tumor is usually clinically evident during the latter part of the second decade; however, reported cases range in age between 4 and 80 years. There is a slight female over male incidence, an almost 2 : 1 site preference for maxilla over mandible, and a marked predilection for the anterior jaws (more than 85% of lesions appear anterior to the second premolar teeth).
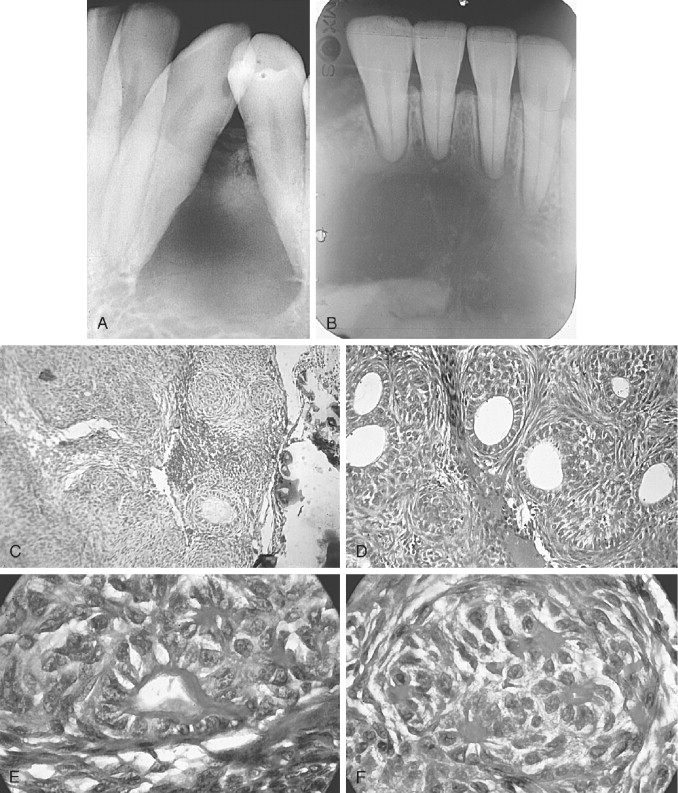
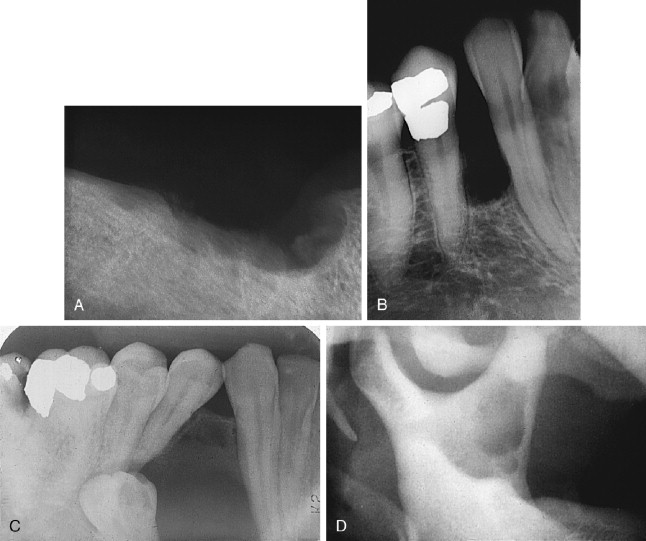
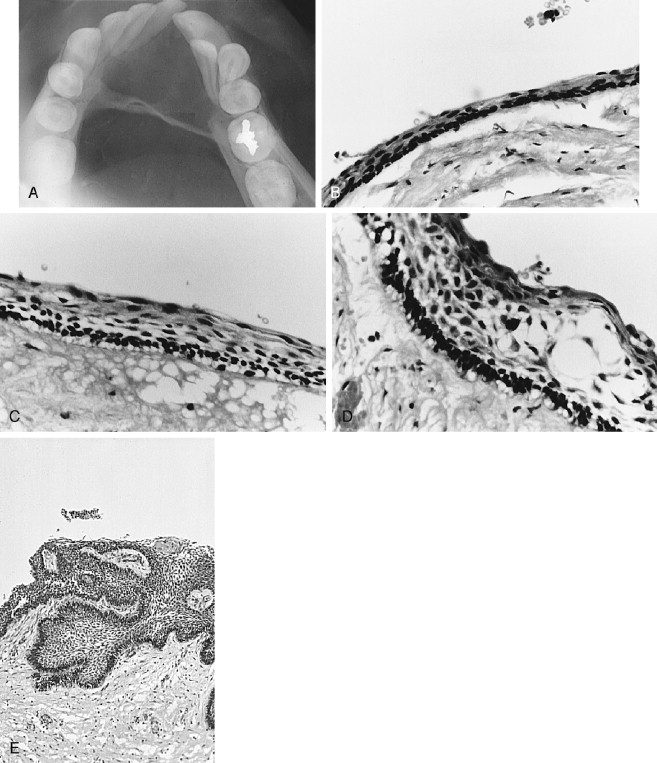
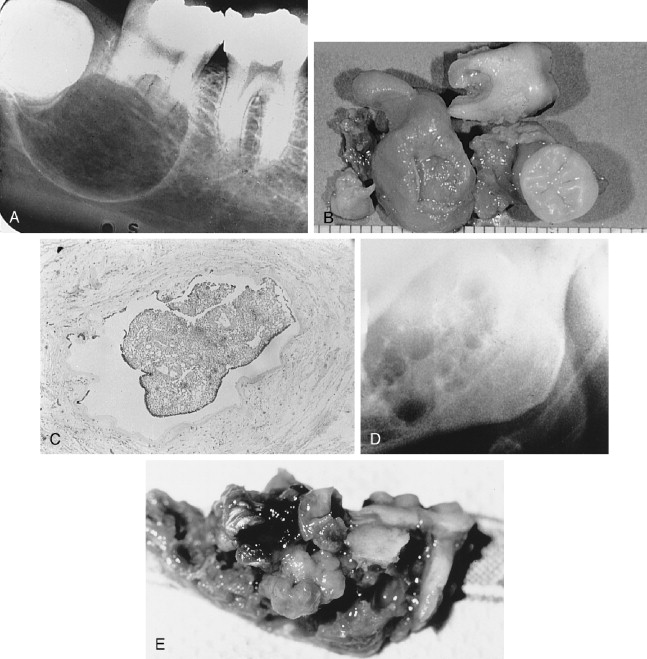
The AOT has several radiographic appearances that may be considered typical but not pathognomonic. The lesion frequently appears as a well-circumscribed, pear-shaped radiolucency (stem portion toward the alveolar surface) lying between divergent roots of the canine and first premolar teeth (or between incisors or premolars); or as a unilocular, radiolucent lesion that is well circumscribed or bounded by a radiopaque margin, lateral to or containing an impacted tooth (similar to a follicular, dentigerous cyst or occasionally a periodontal cyst) ( Figure 25-2, A-H ). Frequently, small, variously shaped faint or marked radiopaque particles (calcifications) may be distributed throughout the lesional space or are concentrated within a small area of a predominantly radiolucent lesion. Rarely, some lesions appear heavily calcified ( Figure 25-1, A , B ). Root resorption occasionally occurs, but is usually not severe ( Figure 25-1, B ).
Histologically, the lesion is usually partially cystic and surrounded by a well-developed connective tissue capsule. The luminal surface is lined with a reduced odontogenic epithelium that leads to thickened mural proliferations of the AOT, which project into and partially or almost completely fill a cystic lumen with tumor lobules ( Figure 25-2, F-H ). The characteristic AOT lesional tissue is composed of a profuse complex of rounded, rosette-like and stream-like aggregates of spindle-shaped odontogenic epithelial cells ( Figure 25-2, G , H ), similar in size and shape to the stratum intermedium cells of the developing tooth germ ( Figure 25-1, C ). Within the epithelial masses, some of the rounded and rosette forms appear to become hollow, assume ductlike or tubelike shapes, and are lined with a single layer of cuboidal or columnar epithelium ( Figure 25-1, D ). Frequently, eosinophilic droplets (likened to enameloid products) are seen within the tubes or lying among the spindled epithelial masses ( Figure 25-1, E , F ). To a greater or lesser degree, calcifications occur within the lumina of the ducts, scattered among the epithelial masses or in the stroma. These often appear as dystrophic irregular masses or have an annular shape, while occasional calcifications have a cementum or dentinlike appearance.
Several cases, personally studied, have demonstrated foci of clear cells within the adenomatoid cellular areas. However, such areas have not influenced treatment. Cases of combined AOT with calcifying epithelial odontogenic tumor (CEOT) have been reported.
Management
Because of site, age, and imaging characteristics, lesions such as periodontal cyst, dentigerous cyst, calcifying odontogenic cyst (COC), calcifying epithelial odontogenic tumor (CEOT), or desmoplastic ameloblastoma can be considered in the differential diagnosis. If sufficient suspicion exists that the lesion could be a CEOT or desmoplastic ameloblastoma, one or several biopsies of the lesion should be performed since treatment varies.
The accumulated world experience with AOT, as reflected in 30 or more years of reports, depicts a lesion in which conservative management produces a uniformly excellent outcome without recurrence, except in one case. Takigami and co-workers reported the case of a 49-year-old woman who had four recurrences of a maxillary AOT, which extended from the maxilla into the maxillary sinus, then extended to the posterior ethmoid and sphenoid sinuses, and finally extended intracranially into the middle fossa with destruction of the sella turcica, dorsum sellae, and anterior clinoid processes. A subtotal resection of the tumor was done.
In almost all instances, the lesion may be removed by surgical enucleation. After reflection of an ample mucosal flap and widened entrance through the usually thinned and expanded cortical bone, the connective tissue capsule of the lesion is encountered. Enucleation is achieved by separation of the lesion from bone without perforating the capsule. Portions of the capsule may be thinned or inflamed, permitting fragments of the lesion to escape the connective tissue envelope. Inspection, irrigation, and gentle curettage of the resultant cavity should remove any residual lesion. Primary closure of the wound with a cavity “filler”(e.g., oxidized cellulose, hydroxyapatite, bone) can be performed. Teeth adjacent to the lesion may have divergently displaced roots ( Figure 25-1, A ) as well as severely resorbed radicular bone. Such teeth should be removed if endodontic treatment and/or “bone fillers” cannot be provided. Impacted teeth in a follicular, dentigerous or periodontal cystic position can occasionally be salvaged for eruption if the lesion can be completely removed without injury to the teeth ( Figure 25-2, A-H ).
SQUAMOUS ODONTOGENIC TUMOR
Squamous odontogenic tumor (SOT) was described and characterized by the collaboration of a small group of pathologists, each of whom had seen previously undescribed examples of the lesion ( Figure 25-3 ). SOT is a relatively rare, benign, but locally infiltrative and occasionally aggressive odontogenic epithelial lesion that appears to have hamartomatous and neoplastic characteristics . Since the initial description, a number of papers have amplified details of the clinical presentations and range of biologic activity of SOT.
Clinicopathologic Considerations
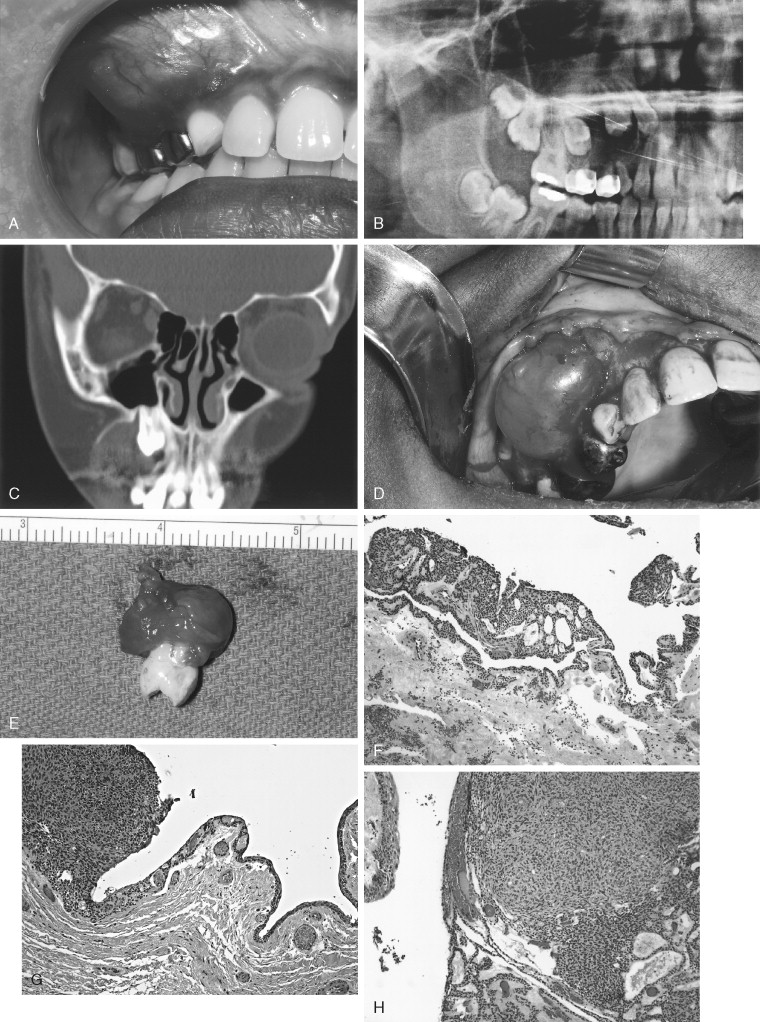
The SOT is found over a wide age range from the second to the seventh decade, with most cases reported in young adults between 19 and 37 years. There does not appear to be a sex or race predilection. The mandible and maxilla are equally affected, and lesions are found singly or multicentrically in all areas of both jaws. The clinical symptoms vary with the circumstances of the individual lesion. Some tumors, within bone only, may be casually discovered by routine radiographs. When lesions occur in dentate areas, alveolar bone resorption around teeth causes mobility and pain. If cortical bone of the mandible or maxilla is penetrated by the lesion, the tumor infiltrates gingiva, occasionally the unattached alveolar mucosa, palatal soft tissue, and rarely the buccal mucosa. Lesions that involve the gingiva as well as alveolar bone, and particularly if multicentric in both jaws, give a generalized impression of severe periodontal disease. Overtly aggressive maxillary tumors may infiltrate the maxillary sinus and nasal floor. An interesting study of familial SOTs in three siblings (two male, one female) has been reported.
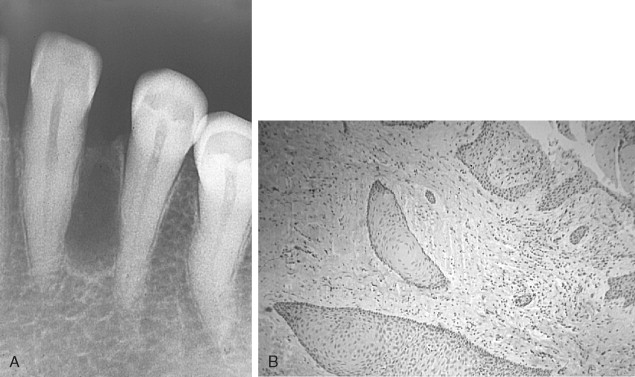
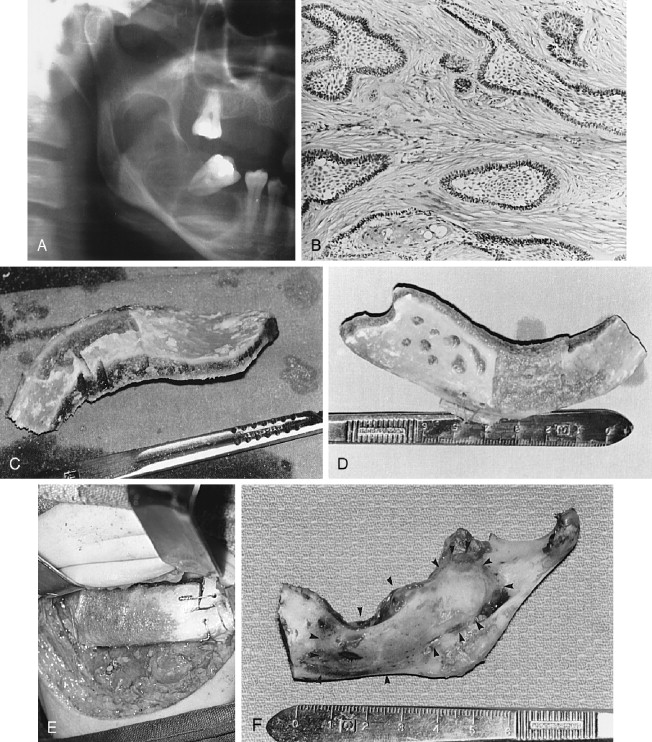
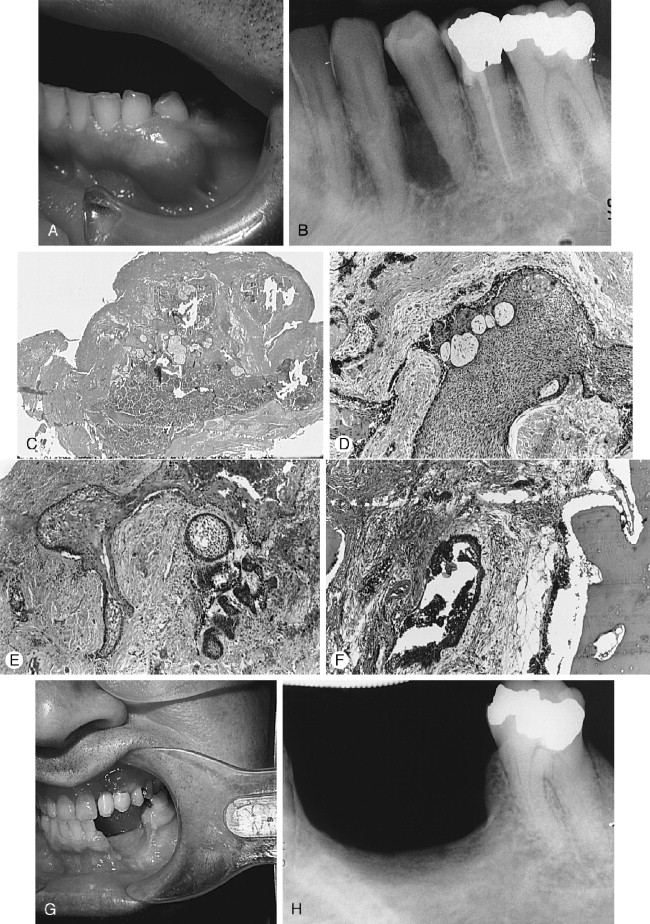
Radiographically, the SOTs, whether as single or multiple lesions of the mandible and maxilla, usually are seen as irregularly shaped or semicircular radiolucent areas with smooth sclerotic borders involving the superior area of the alveolus or roots of teeth. Some lesions are cystic or are associated with cysts and thus may radiographically appear as dentigerous, periodontal, or periapical in form ( Figure 25-3, A ).
Histologic characteristics of solid and cystic tumors have been described. The typical solid lesion consists of numerous islands of squamous epithelium dispersed in a connective tissue stroma. The epithelial islands are variable in size, with irregularly shaped trabeculae that sometimes anastomose as well as rounded masses of benign stratified squamous epithelium with prominent intercellular bridges. The epithelial cells are uniform and without pleomorphism or nuclear hyperchromatism. The central areas of islands occasionally show foci of parakeratin or keratin, calcification, or cellular degeneration. Some epithelial nests show a cuboidal epithelial cell layer bordering the mature connective tissue stroma, whereas most rests have a compressed squamous cell layer lying against connective tissue ( Figure 25-3, B ).
The cystic lesions contain many SOT islands in the connective tissue wall, either completely separated from the odontogenic epithelium lining the lumen or appearing to arise directly from the luminal epithelial lining. Doyle and colleagues (in their study of three cases of SOT) included the first example of a cystic SOT, and Wright (in an excellent study) reported five cystic SOT-like lesions. Hodgkinson and associates, in a report of 79 odontogenic keratocysts found a cyst that initially had been diagnosed and treated as a well-differentiated squamous cell carcinoma arising in a keratocyst. However, during preparation of their paper the diagnosis was revised to SOT in the wall of an odontogenic keratocyst. A case of putative carcinoma developing in a periodontal cyst, reported by Baker and colleagues, appears on close inspection of the photomicrographs, to be an example of SOT in a cyst wall. Van der Waal and co-workers reported the case of a SOT-like lesion in bone, the radiograph of which was compatible with that of SOT islands admixed with epithelial islands resembling ameloblastoma. The published photomicrographs demonstrate why the authors were unable to reach a conclusion regarding the identity of the lesion. Similarly, the senior author (L. Gold) treated a large posterior maxillary dentigerous cyst (radiographic diagnosis) that histologically revealed an odontogenic keratocystic epithelium lining the lumen, with numerous epithelial islands sharing SOT, ameloblastic, and keratocystic histologic characteristics in the cyst wall, and also emanating directly from the keratinizing epithelial lining of the lumen. A retrospective review of a number of lateral periodontal cysts from a study reported in 1973 revealed one that featured SOT-like islands in the cyst wall ( Figure 25-3 ).
Management
There are no, or few, readily recognizable clinical or radiographic signs to presage a diagnosis of SOT. A definitive histologic diagnosis is made after biopsy, or histologic study of the excision specimen, if the lesion is removed without benefit of biopsy. The clinician with a strong index of suspicion may include SOT in the differential diagnosis, if lesions are multicentric in the mandible and/or maxilla, if there is gross alveolar bone involvement and bone loss around tooth roots, and if a generalized clinical picture of periodontal disease is present. However, if lesions are in bone and appear to be radiographically periodontal, dentigerous, or periapical cysts, there is no reason to suspect SOT.
Because SOT is benign but infiltrative, complete excision is required but should be tailored to the clinical and radiographic extent of the lesion. Lesions totally within bone can be removed by a combination of enucleation and curettage under frozen section guidance or can be resected without continuity defect if intact noninvolved bone margins can be established. Involved soft tissue overlying lesions that penetrate cortical bone should be included in the excision specimen, as should teeth within the lesional area.
SOTs in the form of cystic lesions (periodontal, dentigerous, periapical) can be conservatively but completely excised by enucleation and/or curettage and require no further treatment unless histologic study reveals epithelial islands at the border or penetrating the connective tissue cyst wall at the bone interface. Prudence then dictates further excision to a histologically proven safe margin. According to Wright’s report of cystic SOTs, none has recurred, but there are no details offered of cyst wall-bone interface studies of the presence or absence of lesional tissue at the bone interface.
SOT exhibits a biologic behavioral pattern of aggression that appears unaccountable in strictly histologic terms; recurrences of incompletely excised lesions have been reported. Further, since SOT is rare, care must be exercised that the unexpected appearance of multiple islands of epithelium infiltrating the investing connective tissue of the lesion, and with occasional variable histologic characteristics, does not result in a mistaken diagnosis of ameloblastoma or squamous cell carcinoma, as has occurred; or conversely, an erroneous diagnosis of SOT instead of squamous cell carcinoma (see Figure 25-22 ). A squamous cell carcinoma in a bone lesion has been reported in a patient who had multiple SOTs; however, one could speculate whether the lesion was related to the SOT or was coincidental.
CALCIFYING EPITHELIAL ODONTOGENIC TUMOR
Described in 1955 by Pindborg, the calcifying epithelial odontogenic tumor (CEOT) also is known by the eponym Pindborg tumor ( Figures 25-4 and 25-5 ). This seldom-occurring neoplasm has distinctive histomorphologic features completely unlike those of ameloblastoma, to which it has been compared clinically. It is interesting that the 1946 Thoma and Goldman report on the classification of odontogenic tumor shows photomicrographs of one of their cases (case 25) labeled adamantoblastoma, adenoid type (now called ameloblastoma) that is clearly recognizable as a calcifying epithelial odontogenic tumor, as is a case that Ivy (1948) erroneously reported as ameloblastoma.
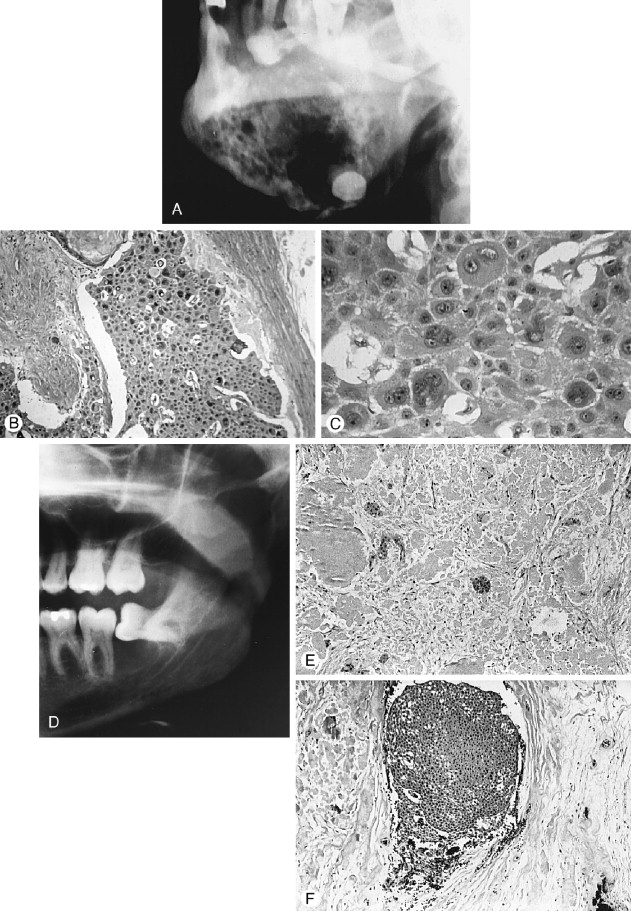
The number of published cases is not large, and the literature expresses conflicting opinions concerning the biologic behavior of CEOT. The lesion appears to occupy a position between hamartoma and aggressive neoplasm; however, there is no question that complete excision of the calcifying epithelial odontogenic tumor is required .
Clinicopathologic Considerations
The calcifying epithelial odontogenic tumor is clinically evident around the age of 40; however, reported cases show an age range between 13 and 80. There is no sex predilection. The mandible is the preferred site over the maxilla in an almost 3 : 1 ratio. The CEOT may occur in any area of the jaw, but the premolar/molar area appears to be favored. The CEOT is slow growing, locally aggressive, and without clinical signs until a clinically large mass is reached or incidental radiographs reveal the lesion. Several extraosseous CEOTs in the anterior gingiva have been described. In addition, CEOT in combination with AOT has been reported.
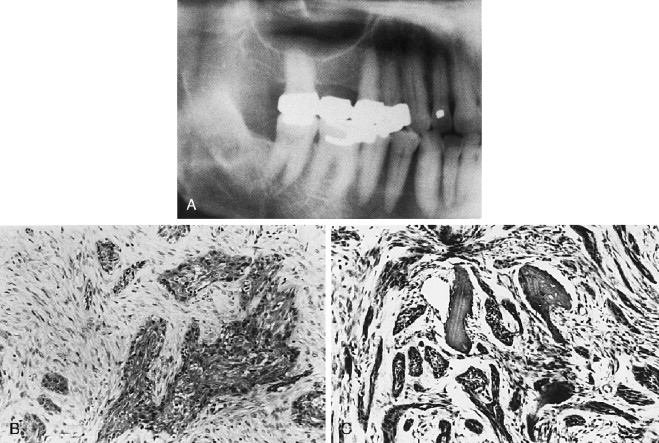
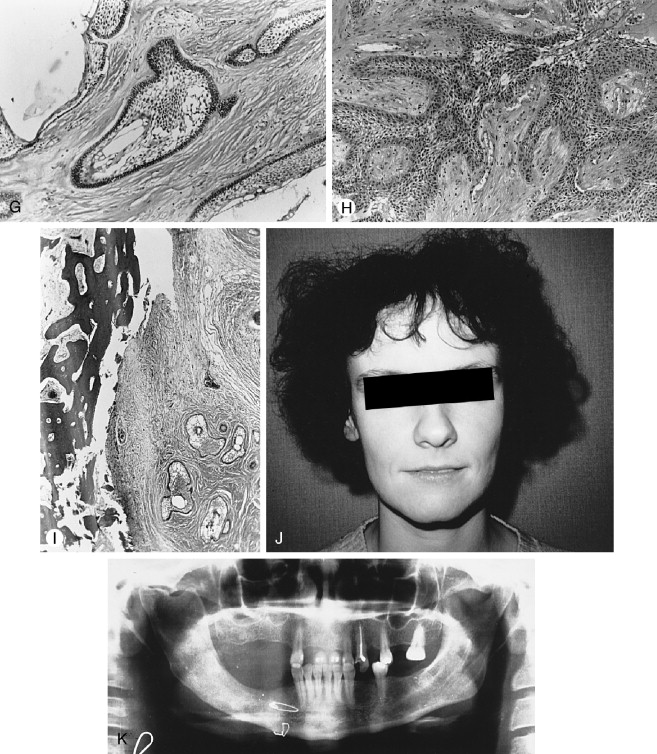
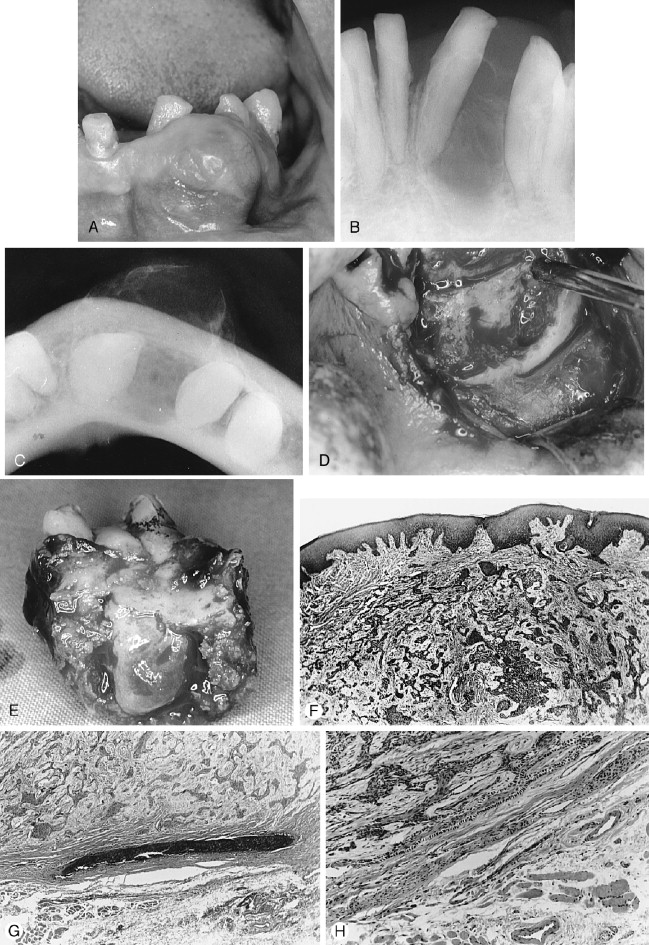
A number of variable radiographic patterns are seen in CEOTs. The lesion may or may not be well circumscribed, mixed radiolucent-radiopaque, unilocular, or associated with an embedded or impacted tooth ( Figures 25-4 and 25-5 ). Infrequently, the lesion is multilocular with bony septae producing a “honeycomb” effect lacking well-defined margins ( Figure 25-4, A ). Calcifications in the lesion appear as scattered, irregular radiopaque small or large particles. If the lesion is heavily calcified, a more dense radiopaque aggregation is produced. Thus, there is no radiographic pathognomonic appearance of the CEOT. Other lesions, such as myxoma, hemangioma, or ameloblastoma, may be suspected if the lesion is radiolucent; calcifying odontogenic cyst and AOT may be suspected if the lesion has radiopacities.
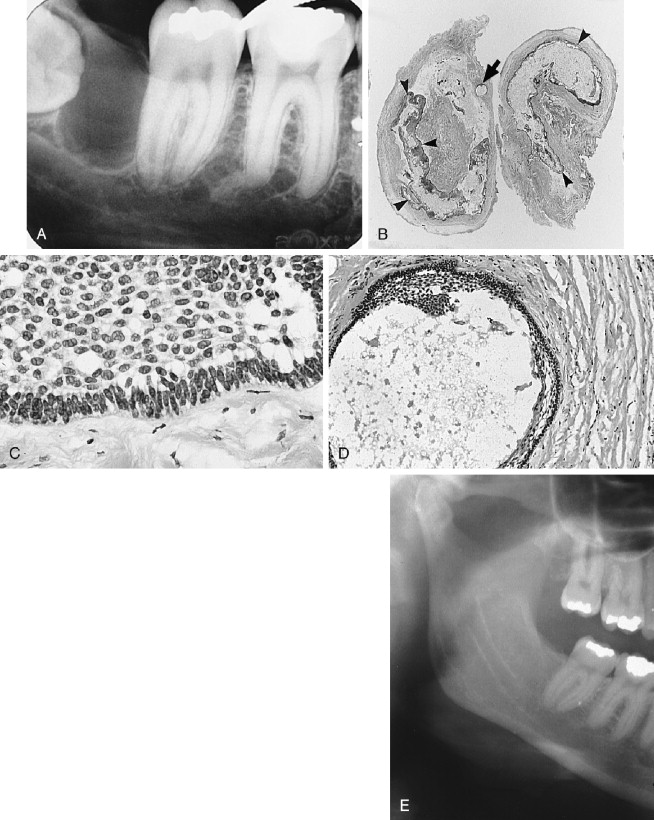
Histologically, the CEOT is characterized by a distinctive picture of trabeculae and/or sheets of large, eosinophilic-staining, squamoid-shaped epithelial cells, frequently with prominent intercellular bridges. The epithelial cells and their nuclei may vary greatly in shape and size. Some nuclei exhibit huge size and odd configurations with large, prominent nucleoli; binuclear and even trinuclear cells may be present. Although bizarre-looking cells are seen, the overall impression is not one of malignancy ( Figure 25-4 , B, C and Figure 25-5 , C). The connective tissue stroma is usually inconspicuous between islands and sheets of epithelium. However, occupying the stroma in small areas of some lesions, or seeming to replace the stroma in large areas of other lesions, is a hyaline-like, homogeneous material identified as amyloid ( Figure 25-4, E , F ). Prominent or scattered calcifications, in concentric crenate-like shapes (Liesegang rings), may occur in the amyloid areas and when present account for the radiopacities seen in radiographs.
Several distinct histologic pattern variants have also been described, the most important of which include clear cell components. Prominent clear cell features in a CEOT appear to be associated with increased aggressiveness of the lesion according to several authors (see later discussions of ameloblastoma with clear cells and clear cell odontogenic carcinoma). An excellent paper by Cheng et al discusses a case of CEOT with a conspicuous clear cell component that also demonstrated marked cytologic atypia of the epithelial islands. Their case had intralesional vascular invasion, numerous mitotic figures, and an increased proliferating index marker (Ki-67) compared to a conventional CEOT. Despite such evidence, the authors did not regard the lesion to be malignant because demonstrable metastases were absent. They further cautioned that there were still insufficient case studies to confirm a generally aggressive behavior in all CEOTs containing clear cells. Yet, Hicks et al , in their 1994 report, record an approximate 14% recurrence rate for conventional CEOT and a 22% recurrence rate for CEOT with clear cell component. The restriction placed by Chang et all on their case is not justified. Their lesion is malignant with or without demonstrated metastasis.
Two more cases of malignant CEOT have been reported. Basu et al (1994) detailed a case with marked nuclear and cellular atypia, perineural invasion around the inferior alveolar nerve, tumor cell invasion of vascular spaces, and metastasis to a submandibular lymph node. Veness et al (2001) presented a case of CEOT that recurred twice with increasing cellular atypia, cellular hyperplasia, and vascular invasion with metastasis to a cervical lymph node.
Management
The 1992 and 2005 World Health Organization (WHO) classification of odontogenic tumors categorizes the CEOT as a benign, locally invasive neoplasm, but does not describe a recurrence rate (i.e. regrowth of residual lesion). Although Hicks et al describe recurrence percentages in their study (see above), a large body of management data has not been compiled, as it has for ameloblastoma, because of the relative scarcity of reported cases and lack of accurate long-term clinical follow-up evaluations.
Perhaps again, as with ameloblastoma, semantics may play a role in the WHO designation of CEOT as benign and locally aggressive. Locally aggressive CEOT does not appear sufficient to describe the aggressive ability of the maxillary lesion described by Bouchaert et al that involved the maxillary sinus, invaded the orbit and ethmoid sinus, and extended to the cranium with subsequent brain compression. Radiographically, lesions may appear well circumscribed or not well circumscribed, but clinically the tumor is infiltrative and not encapsulated.
The surgical treatment of CEOT should be case specific and based on anatomic location and involvement (e.g., maxilla, mandible, pterygoid areas, nasoethmoidal-sinus region), lesion size (volume of jaw involved), clinical activity (penetration of cortical bone, periosteum, muscle, neural involvement), and histocytologic characteristics (neural or vascular infiltration, cellular atypia, marked clear cell presence, mitotic index). As with any pathologic entity, an adequate number of incisional biopsies must be analyzed; extensive lesions should have multiple sites sampled. One must take into account that even the conventional histology of CEOT not infrequently presents a relatively bizarre microscopic appearance (especially to pathologists unfamiliar with this entity).
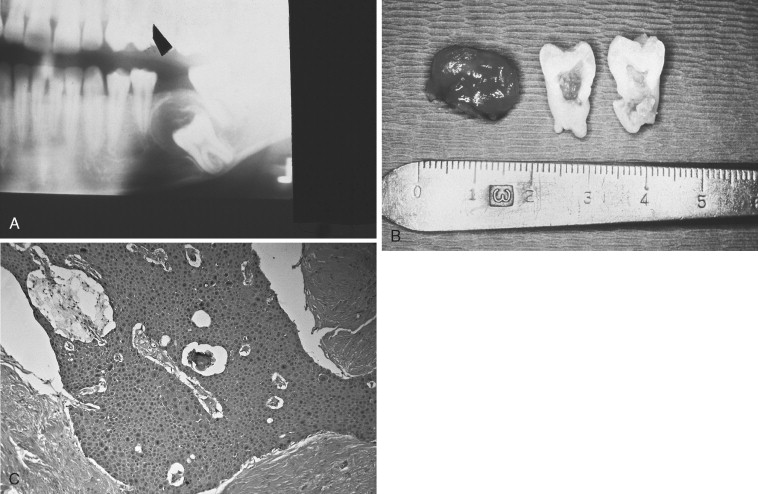
Enucleation with curettage is seldom a treatment option, except for very small lesions (i.e., 1.0 to 1.5 cm). Rs–CD or Rc–CD is more probable to result in cure. Obviously, if the lesion is suitable for resection without continuity defect, and an adequate margin of normal bone can be attained, Rs–CD is the procedure of choice. Caution should be exercised with lesions containing aggregates of clear cells, since clear cell follicles and nests tend to be very infiltrative and may extend further into the marrow spaces in a “skip” manner (i.e. cell nests not anatomically contiguous) and are radiographically not well circumscribed (see Figure 25-21, I and K ).
There are no studies yet that specifically address the issue of “safe” bone margins. Thus a margin of 1.5—2.0 cm may be considered an adequate margin of normal bone, unless frozen sections indicate otherwise. Immediate or delayed reconstruction can be performed as described for ameloblastoma.
AMELOBLASTOMA
The ameloblastoma has more than a 100-year history of recognition, reportage and controversy. Although the most recorded lesion of the odontogenic series, it continues to evoke wide differences of opinion among pathologists and surgeons regarding its position in the neoplastic spectrum, biologic behavior and management. Ameloblastoma has been described as “benign but locally invasive,” “benign and locally invasive with a strong tendency to recur,” and “locally malignant.” Most opening statements of contemporary reports still quote older papers that depict ameloblastoma as a benign and rare, or uncommon tumor. However, ameloblastoma is not rare or uncommon as measured by present institutional statistics, and its benignity is here denied.
The 1971 and 1992 WHO classifications of odontogenic legions categorized conventional ameloblastoma (aka classic ameloblastoma) as a benign but locally invasive epithelial odontogenic neoplasm; and also included a category of malignant epithelial odontogenic carcinomas in which malignant ameloblastoma and ameloblastic carcinoma are separately listed and defined. The WHO described malignant ameloblastoma as a neoplasm in which the metastatic lesion(s) continues to resemble conventional ameloblastoma (i.e., There are no microscopic differences between the metastatic lesion and the conventional ameloblastoma from which it emanates); while ameloblastic carcinoma is described as a malignant tumor in which the lesion demonstrates a spectrum of morphologic/cytologic histologic characteristics of malignancy, and does or does not necessarily metastasize (see later section on Cytologic Malignancy).
The WHO 1971 monograph further proscribed that the designation malignant ameloblastoma should not be applied to ameloblastoma that endangers life by direct extension of the growth into vital structures. It appears that it is permissible to recognize a conventional ameloblastoma as malignant if it does metastasize, and to regard the same ameloblastoma as benign if it does not metastasize!
The senior author (L. Gold) was not alone in objecting to this perplexing reasoning. Several writers had previously faulted the narrow WHO definition of what constituted malignant ameloblastoma or ameloblastic carcinoma on several counts: (1) that a malignant lesion must produce metastases, (2) that the metastases must be histologically identical to the primary tumor, and (3) that the WHO definition is not consistent with malignancy as applied to other neoplasms. Yet, these authors did not reject the implausible WHO definitions, but accommodated to them with further refinements of the original WHO classification.
It is fitting here to quote several writers directly in order to help place this tumor in proper perspective. Willis (a highly respected British pathologist) believed that clinicians have overemphasized the differentiation between benignancy and malignancy in order to gather prognostic information from pathologists regarding excised tumors. He believes that the question “Is this tumor innocent or malignant?,” should be replaced by “ How innocent or malignant is this tumor?” In a specific discussion of ameloblastoma, Willis describes the lesion as “lowly malignant slowly growing” and further writes that “attempts to distinguish between benign and malignant ameloblastoma are futile; they are all malignant in that they are locally invasive and prone to recur.” Carr and Halperin in their paper on malignant ameloblastoma stated in their discussion: “Malignant and benign are poor terms when applied generally to this neoplasm, and the use of one instead of the other seems secondary to an understanding of how the tumor may behave and to an approach to treatment based upon this understanding.”
Consider the following description of the basal cell carcinoma, an acknowledged malignant tumor, offered by Gold : “slowly growing, infiltrative, capable of great destruction of soft tissue and bone, recurrent when not completely eradicated, capable of invading vital structures (orbit, skull, brain), seldom metastatic but capable of metastasis, presenting a number of histologic patterns, and arising from the epithelial skin surface and skin adnexa. If one substitutes oral epithelium for skin surface and dental lamina/enamel organ for skin adnexa, you are describing the known characteristics of the ameloblastoma.” Thus, the ameloblastoma and the basal cell carcinoma share great similarity to each other in their histomorphologic, growth, and infiltrative patterns.
If one takes into consideration that within the past 10 years the steadily increasing number of published reports in the world literature, in addition to the numerous previous accounts over many years that also have described “conventional” ameloblastomas that have metastasized, as well as morphologically and cytologically dysplastic ameloblastomas that have metastasized, one is forced to the conclusion that conventional ameloblastoma is a de novo, low-grade, malignant basaloid tumor with a variable range of histologic patterns, clinical forms and behavior .
In 1999, Eversole revised Elzay’s modification of the WHO classification of epithelial odontogenic tumors to produce his own version; which he (Eversole) considered more refined, but still not entirely satisfactory. Eversole’s thoughtful paper approached but did not reach the essential conclusion that conventional ameloblastoma is a de novo malignancy. Neither did Philipsen and Reichart in their 2002 paper, nor the authors of the 2005 WHO classification, who continue to regard ameloblastoma under benign and malignant categories. None were able to concede the possibility that ameloblastoma is a de novo low-grade malignant lesion.
There are two incontrovertible characteristics that distinguish a malignant lesion: (1) The ability to invade and destroy tissue without regard to anatomic boundaries, and (2) The ability to metastasize. Ameloblastoma, whether in so-called “conventional” or in markedly dysplastic form, qualifies by this definition as a malignancy. The recognition of the de novo malignant status of conventional ameloblastoma permits the progressive sequences from low-grade to high-grade cytologic malignancy to be seen as a continuum. It is possible then to eliminate the artificial and confusing terminologymalignant ameloblastoma and ameloblastic carcinoma—from the WHO classification; ameloblastoma may be described also as a well-differentiated malignancy with or without metastases or poorly differentiated malignancy with or without metastases .
The long-held traditional belief that ameloblastoma is benign, except in rare instances, will be difficult to reverse. It will require more than a casual alteration in mindset for pathologists and surgeons to accept what they have historically and traditionally regarded as a benign tumor, should now be considered a malignancy. However, there are precedents for a change of status among a number of tumors when proof becomes evident, even if, as in the case of ameloblastoma, long delayed (see also morphogenesis of clear cell odontogenic tumor to malignant status in section on clear cell odontogenic carcinoma). A change in perception is required that ameloblastoma, pari passu, is a de novo malignancy and is not a philosophic or nosologic exercise. It removes this lesion from untenable argumentation regarding its pathologic status. It is important to understand that conventional (classic) ameloblastoma, low-grade malignancy not withstanding, is a dangerous lesion precisely because of its unpredictable behavior. This realization would have a salutary effect on the study and management of the lesion.
Clinicopathologic Considerations
The ameloblastoma is asymptomatic and remains undiscovered until lesional growth produces intraoral and/or external jaw swelling, tooth and dental occlusion disturbances, or incidental radiographic examination reveals a lesion. Parasthesia is an uncommon symptom and pain is rarely a presenting symptom unless the lesion causes root resorption and/or tooth mobility. Depending upon the clinical form of the ameloblastoma, the age at which the lesion may become clinically evident is as early as the first decade or as late as the seventh. The lesion may occur in either jaw and in any area, but the sites of predilection are the posterior maxilla and the posterior molar-ramus region of the mandible. The mandible is the favored site over the maxilla by about a 4.5 : 1 ratio.
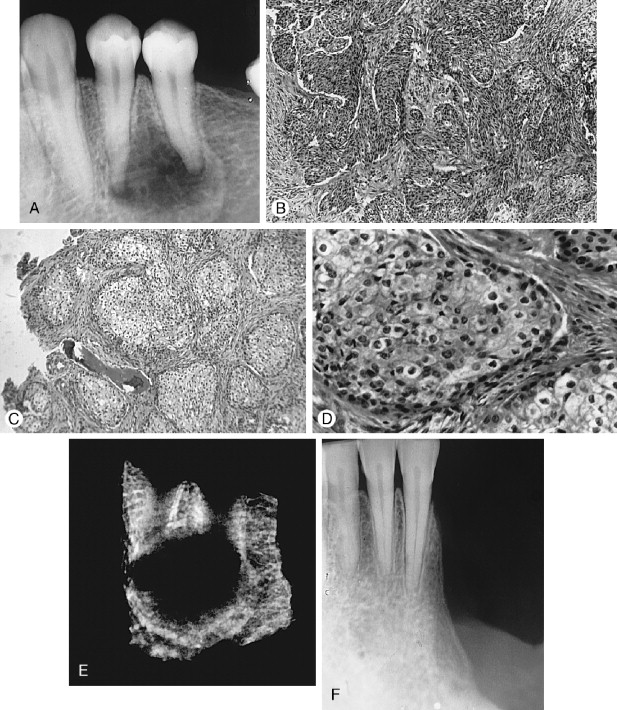
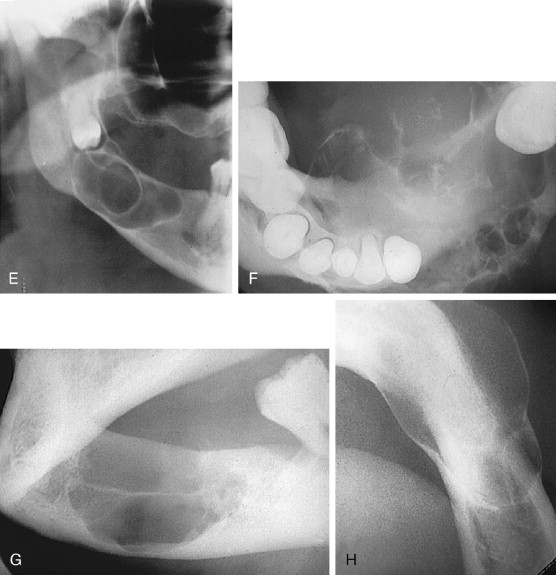
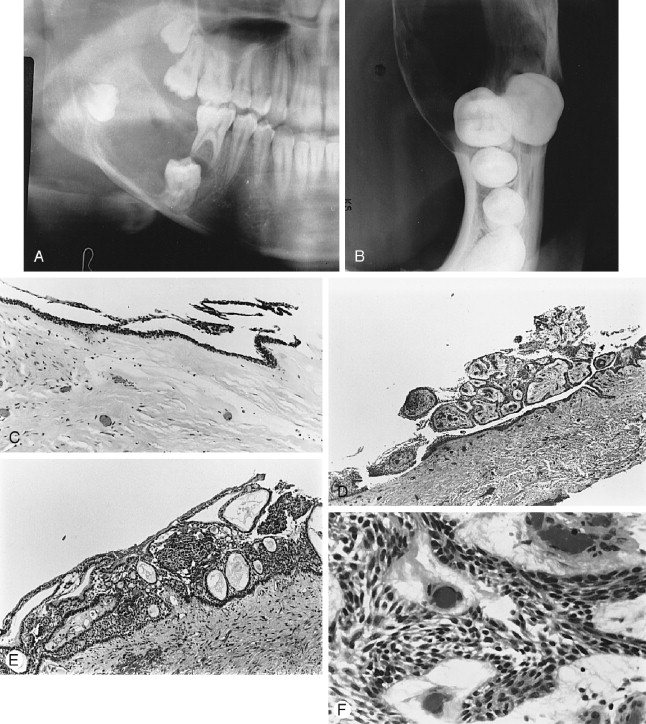
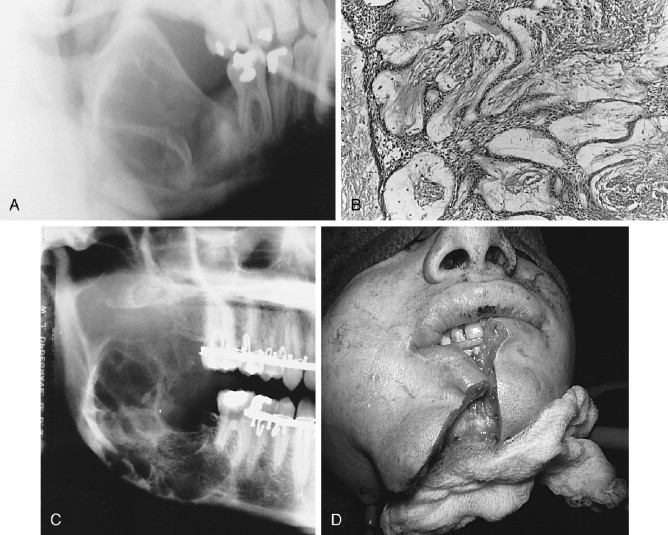
Radiographically, the ameloblastoma presents a unilocular or multilocular radiolucent appearance in several different forms and shapes. The apparent lesional edge may be distinct or indistinct. Small or large unilocular or multilocular lesions may contain unerupted deciduous or permanent teeth and give the appearance of a dentigerous cyst. Lesions in dentate areas cause root resorption and tooth displacement. Expansive distortions of normal mandibular or maxillary anatomic features commonly are caused by this lesion. Maxillary lesions often involve the maxillary antrum and change the normal radiolucency of the sinus to a more opacified appearance. One of the ameloblastoma variants—the desmoplastic ameloblastoma—most often found in the anterior maxilla or mandible, projects as a relatively radiopaque lesion because of the dense connective tissue content. The radiographic image of ameloblastoma is at most suggestive and not pathognomonic; other odontogenic and nonodontogenic lesions may simulate it ( Figure 25-6, A-H ).
Histologically, most ameloblastomas fall within two major morphologic configurations—the follicular and plexiform types. Combinations and variations of these two types, as well as other subtypes, are found even within the same lesion. The follicular type consists of small to large odontogenic epithelial nests (the follicles) and variously shaped and sized ameloblastomatous islands. The plexiform type consists of interlacing strands of narrow or wide odontogenic epithelial trabeculae. Both types have a columnar or cuboidal outer epithelial cell layer bordering the connective tissue stroma and enclosing the inner epithelial cells. The characteristic histologic signatures of the ameloblastoma are the orientation of the outer epithelial cell nuclei, which are positioned (polarized) away from the basement membrane (reverse polarity), and the frequent vesicular appearance of the outer cell cytoplasm abutting the basement membrane. In addition, the inner epithelial cells of the follicles are more or less loosely arranged as in a stellate reticulum, imitating the enamel organ architecture. Not infrequently, the strandular narrow configuration of the plexiform lesion has neither the outer epithelial cell nuclear orientation nor the appearance of inner cell stellate reticulum. A variety of subtypes are built around the basic morphologic characteristics. Compression of the stellate reticulum into a squamoid mass with squamous metaplasia and keratinization produces the acanthomatous type . The inner follicular and outer follicular cells may assume a basal cell appearance throughout the lesion, causing a striking histologic resemblance to basal-cell carcinoma, in which instance the basal cell type is noted. Other ameloblastoma variants also are well recognized such as granular cell, desmoplastic, vascular , and spindle cell (sarcomatoid) types ( Figure 25-7 ).
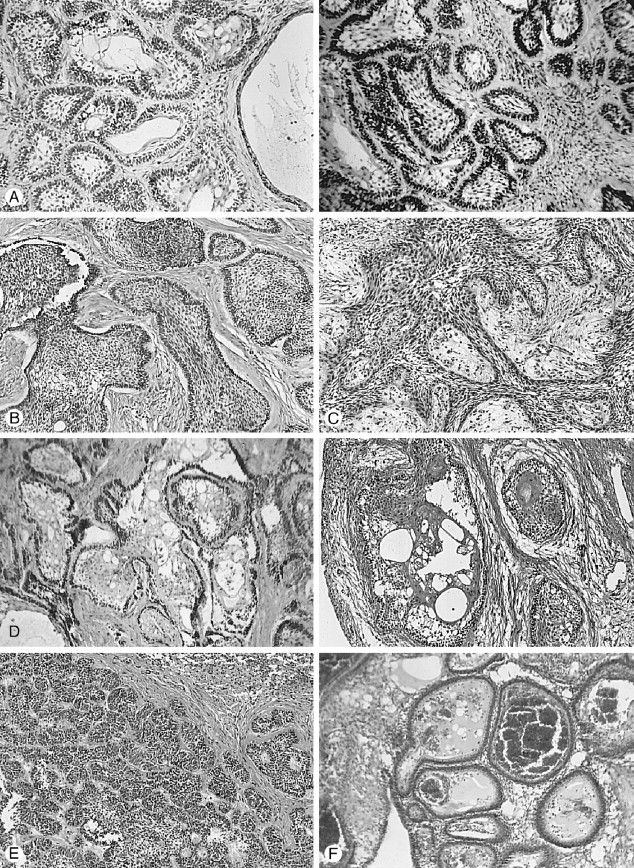
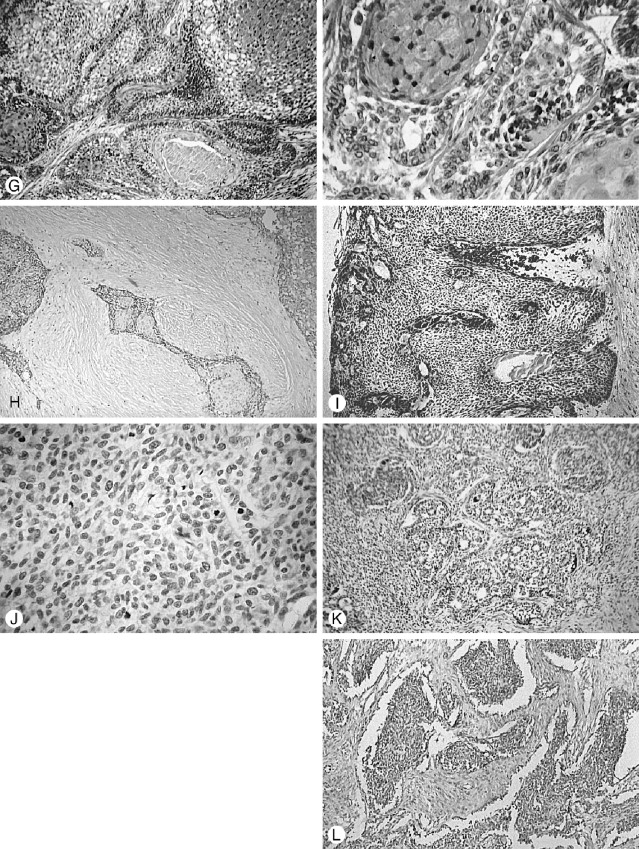
Several rare subtypes have been reported, such as ameloblastoma with “ghost-cell” and keratinization as seen in the calcifying odontogenic cyst. More importantly, however, Waldron et al have reported two cases containing clear cell strands and nests biphasically intermixed with follicular ameloblastoma as clear cell ameloblastoma . Both cases demonstrated marked cytological atypia and one was known to cause the patient’s death by uncontrolled growth and metastasis (see later discussion of clear cell odontogenic carcinoma).
The supporting connective tissue stroma of multicystic and solid ameloblastoma, regardless of histologic subtype, varies from loose to dense, and from scant to plentiful. However, the solid and multicystic forms of the tumor do not produce an effective connective tissue capsule or evoke an encapsulating mechanism by surrounding bone or soft tissue ( Figure 25-7, A-L ). The unicystic ameloblastoma, however, does have a peripheral connective tissue wall, which may be loose or dense, and relatively thin or thick.
Clinical Forms and Mechanisms of Growth
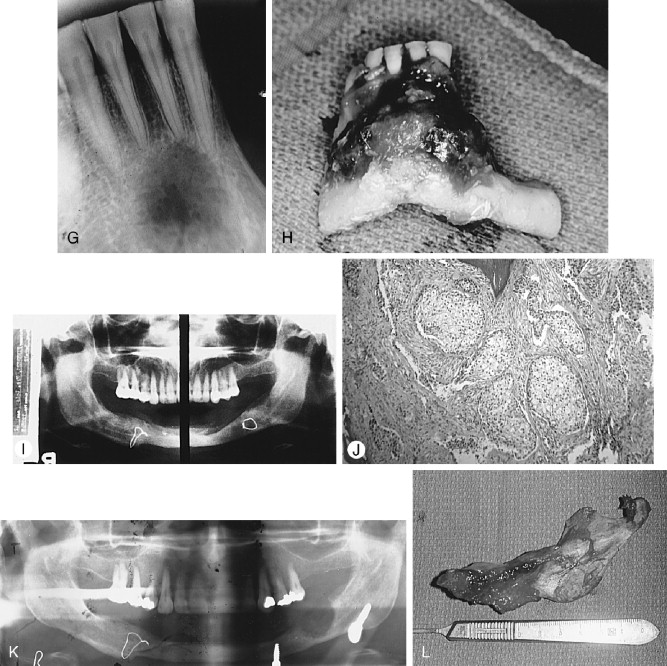
Conventional ameloblastoma presents several clinical (physical) forms as well as histologic types; however, neither physical form nor histologic type is immutable, and combinations of form and type occur. The major intrabony (central) clinical forms are unicystic (unilocular) ( Figures 25-8 to 25-11 ), multicystic (multilocular), and solid ( Figures 25-12 to 25-14 ). The peripheral ameloblastoma is the visibly extraosseous soft tissue form of the lesion found in the gingiva and mucosa of the alveolar process ( Figures 25-15 to 25-17 ).
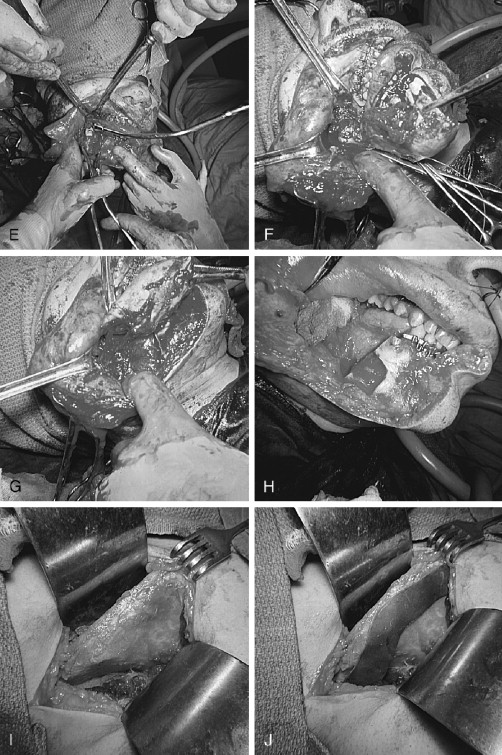
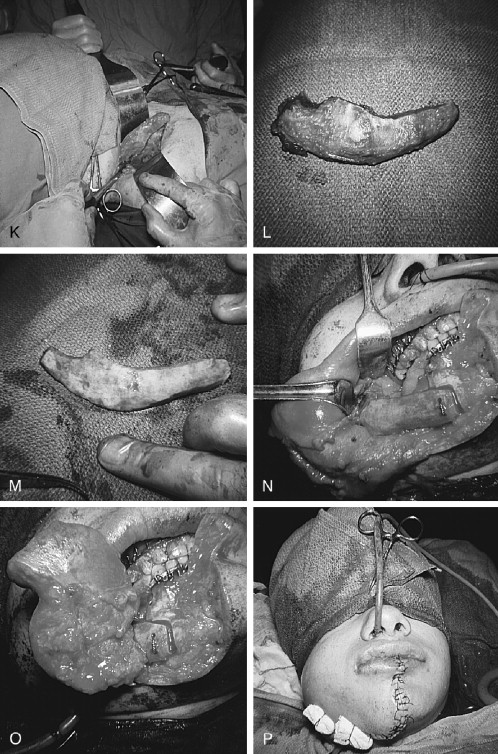
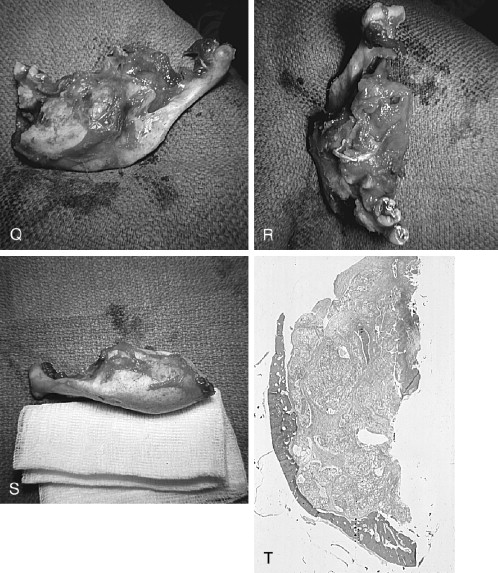
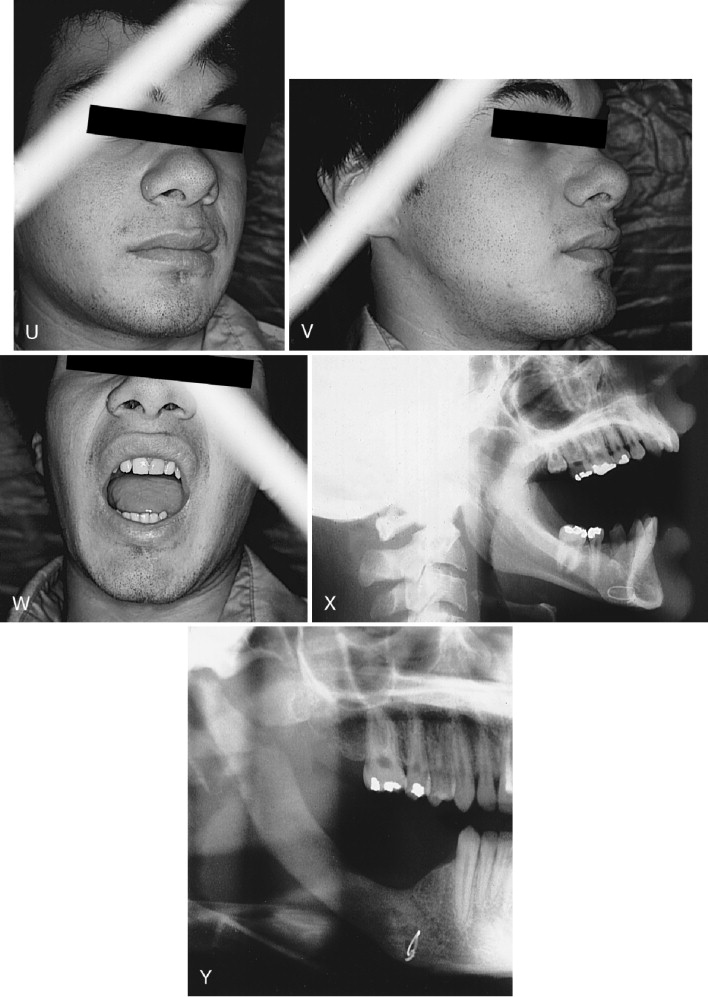
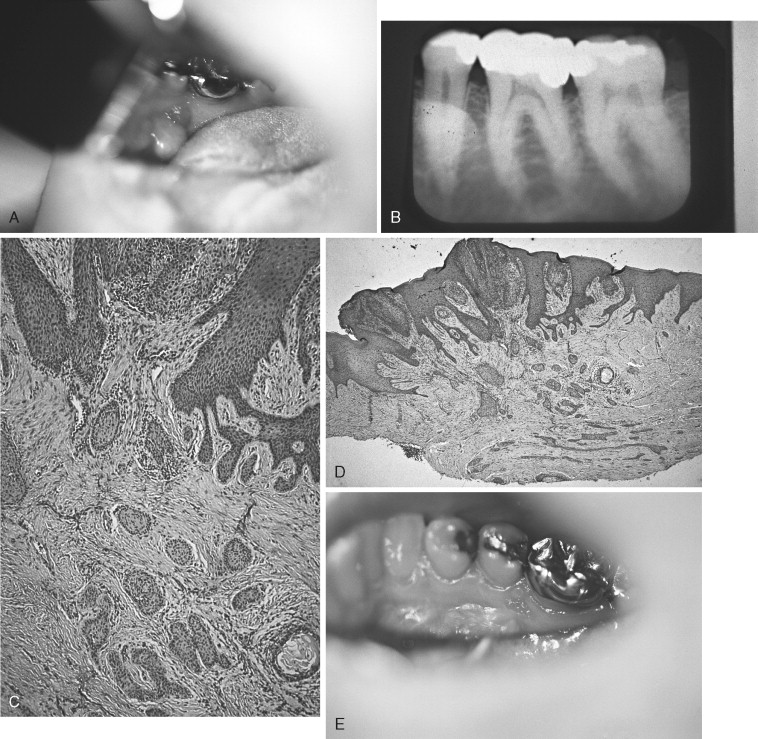
Mixtures of histologic types of ameloblastoma in the original or in a recurrent lesion are not unusual; for example, a unicystic granular cell ameloblastoma, when recurrent years later, showed a multicystic follicular lesion, and a large unicystic lesion simultaneously displayed elements of plexiform, granular cell, follicular, and acanthomatous types.
Unicystic Ameloblastoma.
A unicystic ameloblastoma may form de novo or develop secondarily in an odontogenic keratocyst or dentigerous cyst. The lesion may remain small, become large or even develop into a multicystic form ( Figures 25-9 , 25-11 , and 25-13 ). Occasionally small satellite cysts, separated from the main cystic lesion, may be undetected clinically or radiographically. Small or large unicystic lesions that contain unerupted (impacted) teeth, or those without unerupted teeth, are usually identified radiographically as a dentigerous cyst or odontogenic keratocyst. However, an often-overlooked radiological feature of unicystic ameloblastoma involving a dentate area is the partial resorption of tooth roots (permanent or deciduous), a rare occurrence in dentigerous or odontogenic keratocyst.
The unicystic ameloblastoma exhibits two other notable clinical differences from the multicystic, solid and peripheral forms, besides its physical shape. First, the lesion usually occurs in adolescents, teenagers, and young adults; and second, the lesion exhibits the previously described peripheralencompassing connective tissue wall of varying thickness.
A diagnosis of unicystic ameloblastoma is influenced by several clinical and microscopic decisions: (a) The surgeon may elect to excise a cystic lesion, especially if not extensive, without a preceding biopsy. Thus, a microscopic diagnosis of unicystic ameloblastoma will be unexpected and will occasion further surgery or none, depending upon a proper study of the excision specimen (see laboratory discussion [point d] below). (b) The surgeon may elect to biopsy an extensive cystic lesion; however, unless multiple sites are sampled the diagnosis may not be made, since recognizable microscopic ameloblastic changes may not extend along the entire cyst lining. (c) A correct microscopic diagnosis will not be made if the hospital pathologist fails to recognize the sometimes subtle early or evolving ameloblastic changes described by Vickers and Gorlin ( Figure 25-8 ), or misinterprets a plexiform ameloblastic lining as hyperplastic non-specific epithelium ( Figure 25-10 ). d) Gross sectioning of the unicystic specimen in the laboratory must be done in proper plane orientation so that histologic sections will reveal the ameloblastic proliferation in relation to the cyst lumen, lumen lining and cyst wall. Recognition of the proliferative pattern of the unicystic ameloblastoma has clinical importance. Growth within the cyst cavity ( intraluminal ), growth along the cavity surface ( luminal ), or proliferation into the connective tissue of the cyst wall ( mural ) greatly affects treatment (see discussion under Management).
Solid and Multicystic Ameloblastomas.
Solid and multicystic ameloblastomas may have similar origin in a field of neoplastic change ( Figures 25-12 and 25-13 ). Small foci of ameloblastoma spread out in a three-dimensional manner; the foci become masses and retain a solid form ( Figure 25-14 ) or make microcystic and/or macrocystic spaces lined by ameloblastic epithelium. Intraosseous ameloblastoma grows slowly but inexorably, by invasion of medullary spaces and resorption of cancellous and cortical (compact) bone. Ameloblastic epithelium or lesional connective tissue (stroma) is seen to directly abut resorbing trabecular bone, which still contains endosteal osteocytes and osteoblasts lining the side opposite to the resorbing trabecular surface. There rarely appears to be compensatory bone formation as a reaction to the presence of the tumor. Osteoclastic cellular activity is very rarely seen microscopically. The often cited “impenetrable barrier” of the compact (dense) bone of the mandibular inferior border is mythical. The inferior border is resorbed by the tumor, sooner or late, dependent upon the growth vectors of the lesion.
The pathophysiologic mechanism of the resorptive process is not yet completely known; however, contributing factors have emerged. In 1989, hypercalcemia was reported in two patients with metastasis of ameloblastoma to the lungs, thus suggesting evidence of an osteolytic humoral factor. A report published in 2004 by Abdelsayed et al found that a parathyroid hormone-related protein (PTHrP) was expressed by all lesions (100%) in a study of 30 unicystic and multicystic ameloblastomas. This local expression of PTHrP by tumor cells may readily provide a humoral cause of bone resorption and aggression by the tumor. Other genetic and molecular studies have contributed and will contribute to further understanding of this tumor.
Peripheral Ameloblastoma.
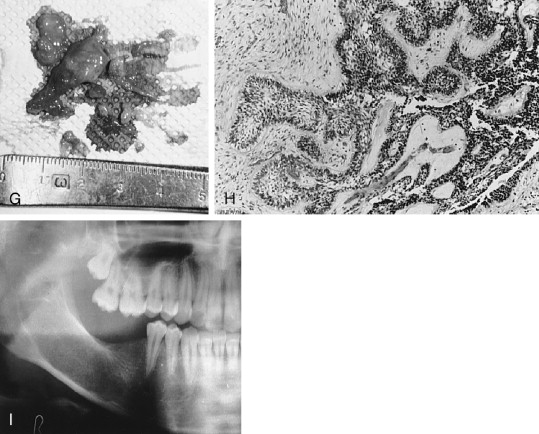
The peripheral ameloblastoma (by definition) is presumed to originate from residual or displaced odontogenic epithelial tissue in the gingiva or mucosal surface overlying the alveolar process ( Figures 25-15 and 25-16 ), and is the soft tissue counterpart of the intraosseous ameloblastoma, occurring on the gingival and mucosal covering of the alveolar process of the jaws. The lesion may present a smooth sessile shape if the tumor is contained entirely within connective tissue well below the surface epithelium, and a warty or papillomatous shape if the lesion infiltrates the surface epithelium. Like the intrabony ameloblastoma, the peripheral ameloblastoma is not encapsulated but is strongly circumscribed by stromal connective tissue through which it can infiltrate surrounding mucosa as well as penetrate periosteum and involve bone . The peripheral tumor has a basaloid pattern primarily, but frequently is mixed with follicular and acanthomatous elements, and in the absence of other subtypes appears identical to basal cell carcinoma. Even a clear cell variant has been described. Some lesions appear to emanate from surface epithelium or fuse with the surface ( Figure 25-16, F ). Radiographs of the alveolar process contiguous with the lesion show varying involvement of the underlying bone and occasional resorption of adjacent tooth roots. The underlying bone is occasionally resorbed medial to the lesion or sometimes appears cupped-out by a pressure phenomenon ( Figures 25-15 and 25-16 ). There is no certain knowledge that the lesion cannot simultaneously originate from bone and soft tissue and grow centrifugally rather than centripetally. For a discussion of further potential links between peripheral ameloblastoma and intraosseous ameloblastoma, the reader is referred to papers by Ide et al and Gold.
The peripheral ameloblastoma, because of its surface accessibility is the most readily managed of the ameloblastomas by relatively conservative surgery. However, growth characteristics such as tumor extension to the surface and medial and lateral infiltrating growth with circumscription but without true encapsulation must be taken into account. Recurrence, although uncommon, has been reported. ( Figure 25-17, A-E ) (see discussion under Management).
Philipsen et al, in a review of 160 cases of peripheral ameloblastoma culled from the world literature, identified 6 cases reported as malignant variants. These lesions were described as basal cell carcinoma or squamous cell carcinoma. One of the 6 cases was excluded (because of incomplete documentation) by Wattan et al in a paper that also reviewed reported malignant peripheral ameloblastomas, and discussed their own cases of recurrent peripheral ameloblastoma with severe dysplasia.
In a letter to the editor in 2004, Ide and Kusama discussed a paper published by them in 2001 of a maxillary peripheral ameloblastoma which “in spite of seemingly innocuous histology, showed unusual mitotic activity.” The lesion was described as “partially malignant.” In 2003, they studied an excised submandibular lymph node from the same patient, which was diagnosed as metastatic well-differentiated peripheral ameloblastic carcinoma secondary to the original lesion. Ide and Kusama now suggest that, in view of this retrospective diagnosis, mitotic activity in a peripheral ameloblastoma is possibly correlated with malignancy. They also find a corollary between the “conventional” appearance of peripheral ameloblastoma and “conventional” intraosseous ameloblastomas that have metastasized.
Their excellent discussion demonstrates the dilemma faced by pathologists who are reluctant to diagnose a lesion as malignant unless there is clear morphologic and cytologic evidence of malignancy, but also may represent an overly cautious desire not to overdiagnose a lesion, which may influence a surgeon to perform what may be unwarranted, aggressive surgery.
Cytologic Malignant Ameloblastoma.
As previously argued in the introduction to the section on ameloblastoma, the authors consider conventional ameloblastoma a low-grade de novo malignant tumor. Numerous reports document conventional ameloblastomas that aggressively invade adjacent and regional tissues as well as metastasize to the bronchopulmonary system, local and distant lymph nodes, and distant organs. Accounts suggest that nothing distinguishes some metastatic lesions from conventional ameloblastoma, but several writers have commented that a number of photomicrographs reveal pronounced histologic dysplastic differences from conventional ameloblastoma that should have been recognized.
There is a tendency among some pathologists, once the histologic diagnosis of ameloblastoma is made, to dismiss the lesion from further consideration, since common knowledge holds that all ameloblastomas, of whatever subtype, behave in a similar manner. However, it is reasonable to expect that increased clinical aggression beyond the apparent slow progression of conventional ameloblastoma will be accompanied by histologic evidence of cytologic or gross atypia. It is probable that subtle morphologic histologic alterations are not recognized or are dismissed as unimportant, and obvious and blatant evidence of increased cellular atypia is regarded as “atypical,” rather than an extension of the lesion’s know biologic potential.
Subtle cytologic mutations can occur in ameloblastoma without physical or symptomatic awareness. However, sudden, rapid, intraoral expansion of bone or soft tissue, and marked facial or cervical swelling, often accompanied by sensory loss and/or pain, may signal significant morphologic and cytologic changes in a lesion . Imaging (panographic, CT, MRI) also may show extensive jaw bone destruction.
In unusual instances, clinical examination and imaging of maxillary lesions may reveal tumor masses, which extend into the maxillary sinus, nasoethmoidal, and pterygoid spaces or the skull base. Mandibular lesions may infiltrate the floor of the mouth, parapharyngeal, and cervical regions. A clinically symptomatic patient may be the first sign of the presence of a de novo ameloblastic cytologic malignancy. However, more typically they will be patients who have been treated previously, sometimes years before, or have had numerous, intermittent unsuccessful procedures .
In addition to imaging the primary jaw lesion when cytologic malignancy is proved or suspected, CT and PET examination should be extended to the chest, and bone scan to the skeletal system. Isolated and multiple lung and pleural metastases as well as vertebral, hip, and other bone lesions may occur. Metastases to brain, kidney, and even myocardium have been reported. Metastases to the skull, spine, lymph nodes, and distant organs do not occasion dispute concerning their metastatic credentials. However, metastasis to the lungs, often recorded as a favorite site, has raised the suggestion that aspiration occurred during a surgical procedure. Although implantation can and perhaps does occur, given the protective measures used to guard against aspiration, it must be a rare event measured against the numerous reports of metastases to the lungs. MacIntosh has voiced the convincing argument that a common site of metastasis of oral salivary gland malignancy (e.g., mucoepidermoid carcinoma) is also to the lung, about which there has been no question of validity ( Figure 25-18 ).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


