Fig. 10.1
Normal tissue complication probability curves (NTCP) as a function of the mean PG dose for Michigan (dashed line) and Utrecht (solid line). Clinical NTCP values (using mean dose bins of 20 Gy) are shown for Michigan (open squares) and Utrecht (black squares), including 95 % confidence intervals (Reproduced with permission from Dijkema et al. [7])
Van Luijk et al. [9] have suggested, in a preclinical study, that there is a differential PG dose response dependent on the distribution of RT dose across the PG. Irradiation of the whole PG, with what is regarded as a sub-tolerance dose of 10 Gy, did not result in functional loss (recovery of salivary function). However, when 10 Gy is delivered to the entire PG (“bath dose”) and an additional 30 Gy is delivered to a smaller sub-volume (“shower dose”), specifically to the caudal half of the gland, then this resulted in greater functional loss. Conversely the administration of the shower dose (30 Gy) to the cranial half of the gland was shown to not cause additional functional impairment [10]. Buettner et al. [11] showed that a radiobiological model describing the distribution of dose across the PG was better than a mean whole gland dose parameter alone for predicting physician-graded late xerostomia (LENT-SOMA).
How IMRT Improves Radiation Dose Delivery
The delivery of therapeutic radiation for HNC has changed significantly over the last two decades. The original technique of conventional 2D radiotherapy (2D-RT) delivered a homogenous dose to both the malignant and normal tissues using paired, opposed radiation beams with limited ability to shape the dose distribution (Fig. 10.2). This led to a very high frequency of early and late normal tissue toxicity [12]. The first RT technique that was investigated to reduce SG toxicity, specifically PG dysfunction, was 3D conformal RT (3D-CRT). This technique uses a multi-leaf collimator (MLC) comprising many narrow, mobile lead leaves, to shape the radiation beam and produce convexities in the dose distribution; however, it is not possible to produce concavities.
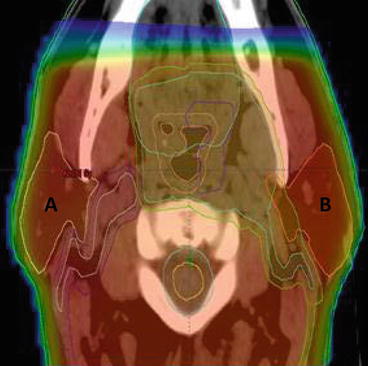

Fig. 10.2
Dose distribution with a 2D-RT treatment plan for oropharyngeal carcinoma. A right PG, B Left PG, red shading region receiving >95 % of prescribed radiation dose
Intensity-modulated radiotherapy (IMRT), first described in 1997 [13], is an advanced form of 3D-CRT with the MLC used to define the radiation dose intensity independently for different regions of the target volume. This is achieved by using multiple beam directions, commonly five or seven equi-spaced fields. The shape defined by the MLC is then varied over time. The most frequent methods used are step and shoot, with multiple static fields of different shapes or dynamic MLC with continuous, automated movement of MLCs without treatment interruption. IMRT may also be delivered using arc therapy delivering IMRT with one (360°) or two (720°) continuous rotations of the radiation source around the patient. Examples such as VMAT (Elekta), RapidArc or tomotherapy have the main benefit of a reduction in treatment time [14].
IMRT will therefore define concave and convex shapes (Fig. 10.3) thus allowing high-dose treatment of tumour sites but avoidance of adjacent nontarget normal tissues. The use of IMRT means that delineation of the target and nontarget tissues, patient immobilisation and verification of patient and tumour positions during a course of treatment become even more important. This is to avoid missing the edge of the tumour, which may lead to an increase in recurrence rates (see section “Local disease control with PG-or SMG-sparing IMRT”) with possible overdose of normal tissues.
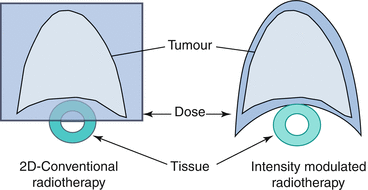

Fig. 10.3
Schematic diagram showing the improvement in conformality achieved with IMRT (right) vs. 2D-RT (left)
In addition to the reduced normal tissue toxicity, IMRT has the potential to allow dose escalation to the tumour which will increase cell kill and may improve recurrence and cure rates. Dose escalation has been studied recently in a number of phase I/II studies [15–18], and a multicentre phase III RCT is currently recruiting in the UK [19].
Non-randomised Studies of PG-Sparing RT and IMRT for the Treatment of Head and Neck Cancer
In an early planning study where patients were treated with unilateral irradiation of the neck lymph nodes, 3D-CRT was shown to be superior to 2D-RT for both target volume coverage and contralateral PG sparing [20]. In addition, when treating the bilateral neck, a reduction in the mean radiation dose delivered to the contralateral PG (21 Gy vs. 58 Gy) was reported in a study by Eisbruch et al. [21] using 3D-CRT.
Eisbruch et al. subsequently pioneered the implementation of IMRT for the routine treatment in HNC. Their initial case series of 88 patients treated with IMRT reported that the PG mean dose should be limited to 26 Gy or 24 Gy, for stimulated or unstimulated flow, respectively, to maintain a substantial fraction of pre-IMRT PG saliva flow [22]. Furthermore they showed that patient-reported xerostomia significantly improved over time with the use of IMRT [23].
Further single-arm phase I and II prospective studies have shown that PG-sparing IMRT produces favourable xerostomia rates for several common subsites of HNC (Table 10.1).
Table 10.1
Summary of prospective single-arm studies (by subsite), with xerostomia end points where PG-sparing IMRT is used for the treatment of head and neck cancer
|
Author (year)
|
Disease site
|
Total (n)
|
Months follow-up
|
Xerostomia end point
|
Frequency (%) or grade of end point
|
|---|---|---|---|---|---|
|
Lee et al. (2009) [24]
|
Nasopharynx
|
68
|
31
|
≥grade 2 (RTOG)
|
13.5 %a
|
|
Marucci et al. (2012) [25]
|
Nasopharynx
|
31
|
24
|
≥grade 2 (RTOG)
|
75 %b (5-field plan)
|
|
44 %b (7-field plan)
|
|||||
|
Mean total
|
20.5b (5-field plan)
|
||||
|
XQ score
|
18.5b (7-field plan)
|
||||
|
Hunter et al. [26]
|
Oropharynx
|
72
|
24
|
Mean xerostomia score (CTCAE)
|
1.0a
|
|
Eisbruch et al. [27]
|
Oropharynx
|
69
|
24
|
≥grade 2 (RTOG)
|
67 % (6 m)
|
|
25 % (12 m)
|
|||||
|
15 % (18 m)
|
|||||
|
16 % (24 m)
|
|||||
|
Richards et al. [28]
|
Unknown primary
|
19
|
23.7
|
≥grade 2 (LENT-SOMA)
|
29.4 % (6 m)
|
|
14.3 % (12 m)
|
|||||
|
Miah et al. (2010) [15]
|
Larynx and hypopharynx
|
60
|
51.2 (DL 1)
|
≥grade 2 (LENT-SOMA)
|
9 %a (DL 1)
|
|
36.2 (DL 2)
|
8 %a (DL 2)
|
||||
|
Toledano et al. (2012) [29]
|
Mixed SCCHN
|
208
|
25.3
|
≥grade 2 (RTOG)
|
16 % (18 m)
|
|
Scrimger et al. (2007) [30]
|
Mixed SCCHN
|
64
|
48
|
Mean total RTOG score
|
1.1a
|
|
Munter et al. (2004) [31]
|
Mixed SCCHN
|
18
|
23
|
≥grade 2 (RTOG)
|
17 % (>3 m)
|
|
Zaidi et al. (2011) [17]
|
Thyroid
|
45
|
12
|
≥grade 2 (LENT-SOMA)
|
35 % (DL 1, 0–3 m) 65 % (DL 2, 0–3 m)
|
RCTs of PG-Sparing IMRT
RCT of Conventional RT Versus IMRT
Four phase III randomised controlled trials (RCTs), one multicentre and three single institution, have compared conventional 2D-RT with IMRT.
The PARSPORT trial [32] is the largest study to investigate PG-sparing IMRT in non-nasopharyngeal carcinoma (NPC) patients; it is also the only multicentre IMRT RCT for squamous cell HNC. Ninety-four patients from six UK centres were randomised to IMRT vs. 2D-RT. The primary end point was patient-reported high-grade (≥G2) xerostomia by the LENT-SOMA scale at 12 months after RT. The secondary end points were global and xerostomia-specific quality of life scores, acute and other late radiation side effects, measurable PG and floor of mouth salivary flow and progression-free and overall survival. The salivary function outcomes at 12 months for contralateral measurable PG salivary flow were 47 % for IMRT compared to 0 % for conventional RT (p < 0.0001). The frequency of high-grade xerostomia was 38 % for IMRT and 74 % for conventional RT (p = 0.0027) (Fig. 10.4). In addition, the EORTC-HN35 subscale score for dry mouth showed deterioration from baseline (increased mean score) at all time points after RT. At 12 months the mean increases were 56.6 (2D-RT) and 48 (IMRT), and at 24 months the mean increases were 59.3 (2D-RT) and 34.8 (IMRT). Despite numerical improvement in the IMRT arm when compared with the 2D-RT arm, the result was not statistically significant (p > 0.01) (Fig. 10.5).
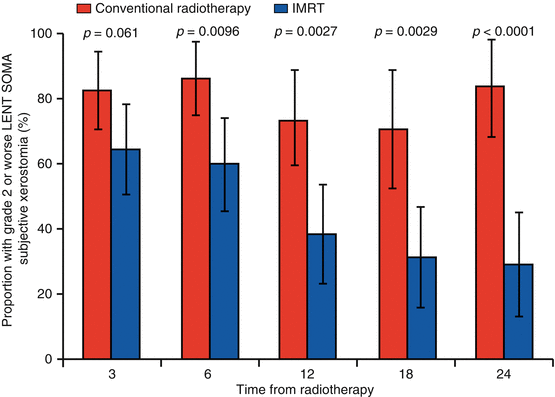
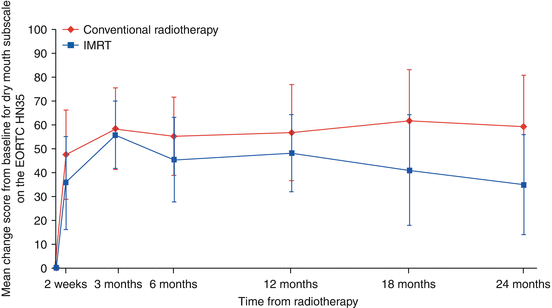

Fig. 10.4
Proportion of patients reporting grade 2 or worse LENT-SOMA subjective xerostomia salivary gland side effects (primary trial end point ≥ grade 2 subjective xerostomia at 12 months) (Reproduced with permission from PARSPORT trial [32])

Fig. 10.5
Mean EORTC QOL HN35 dry mouth subscale score changes from baseline (Reproduced with permission from PARSPORT trial [32])
Peng et al. [33] published the most recent and largest RCT investigating PG-sparing RT techniques. Patients with NPC and no distant metastases were randomised to IMRT (n = 315) or 2D-RT (n = 325). Administration of induction, concomitant and/or adjuvant CT was similar between treatment groups. The 6-month rate of xerostomia (any CTCAE grade) showed a significant benefit for IMRT vs. 2D-RT, 39.5 % vs. 99.4 % (p < 0.001).
Two smaller, single institution RCTs are reported for patients with early-stage NPC [34,35]. Pow et al. [34] (45 patients) reported stimulated whole mouth saliva (WMS) and unilateral stimulated parotid saliva (SPS) flow rates. At 12 months post-RT, the proportion of patients with stimulated WMS and SPS flow recovery to >25 % of the pre-RT level was significantly higher with IMRT vs. 2D-RT (stimulated WMS 50 % vs. 4.8 % and SPS 83.3 % vs. 9.5 %). Despite this finding, patient-reported oral quality of life (EORTC-HN35) scores for sticky saliva and dry mouth were not significantly different between the two techniques; this may be related to the small number of patients enrolled in the trial.
Kam et al. [35] (60 patients) reported a lower proportion of patients with ≥ G2 physician-reported xerostomia (RTOG) using IMRT compared to 2D-RT (39.3 % vs. 82.1 %, p = 0.001) at 1 year. This was comparable to the PARSPORT trial outcomes. As with Pow et al., the fractional recovery of flow from baseline for stimulated WMS, 0.41 vs. 0.2 (p = 0.01), and SPS, 0.9 vs. 0.05 (p < 0.001), was significantly better at 1 year posttreatment with the use of IMRT.
A systematic review has been published recently by O’Sullivan et al. [36] which assessed the benefit of IMRT over conventional 2D-RT for multiple adverse effects and disease outcomes and specific to this discussion, xerostomia.
They retrieved seven prospective, retrospective and case-controlled studies with xerostomia as an end point, which enrolled 567 patients between them. Five of the studies reported a statistically significant reduction in xerostomia at 6 months [37], 1 year [32,34,35] or 20 months [38] after RT. However, two other studies [39,40] showed no significant difference in xerostomia outcomes.
The authors concluded that if a reduction of xerostomia and an improvement in quality of life are the main outcomes of interest, then IMRT is the recommended treatment for all nasopharyngeal, oropharyngeal, hypopharyngeal, laryngeal, oral cavity and unknown primary cancers when delivery of RT to lymph node regions, requiring inclusion in the treatment volume, would result in irreparable damage to SG function, if a less conformal RT technique is used due to their inability to maintain SG doses within their tolerance limits.
RCT of 3D-CRT Versus IMRT
Gupta and colleagues [41] report the only RCT of PG-sparing IMRT vs. 3D-CRT. Sixty patients were assessed for radiation dosimetry and physician-reported SG toxicity (RTOG, acute and late). The ≥ G2 acute SG toxicity using IMRT was lower, 59 % vs. 89 % (p = 0.03) and for late toxicity ~30 % vs. ~75 % (p = 0.001, data in histogram figure only). IMRT plans achieved higher dose conformality and PG sparing. The mean (95 % CI) contralateral PG dose for 3D-CRT vs. IMRT plans was 49.8 Gy (46.5–53.1 Gy) and 28.8 Gy (27–30.7 Gy), respectively.
Implementation of IMRT: National and International Experience
The implementation of IMRT into routine practice is a meticulous process requiring multidisciplinary teamwork to provide the safe and precise delivery of this highly conformal RT technique [42]. For implementation and ongoing routine treatment, the clinical/radiation oncologist, physicist, dosimetrist and therapy radiographer must work closely together. Once the primary tumour site of interest has been determined, a stepwise implementation plan should be developed [42].
Firstly, treatment planning requires reproducible immobilisation of the patient, commonly using a thermoplastic shell. IMRT no longer necessitates a straight cervical spine therefore allowing extension of the neck which can reduce dose to critical normal tissues. The departmental set-up errors and patient movements in the treatment position should be audited to determine margins that should be added to the outlined regions of tumour and normal tissue. Patients should be imaged in the treatment position with axial scans, currently CT+/−MRI or PET, and the tumour and normal tissues delineated using computerised planning software performed by an oncologist. Alongside this a quality assurance programme should be maintained to confirm the accuracy of IMRT treatment delivery, planning systems and software. Guidelines for the roles of each team member in the delivery of IMRT are outlined in a recent joint ACR and ASTRO publication [43].
UK – A survey of the use of IMRT in the UK was performed in 2008 [44]. Fifty of 58 UK centres responded (~89 % of all patients treated) with 46 of 50 centres having at least 2 IMRT-capable treatment machines but only 18 centres treating patients with it. Despite HNC and thyroid cancer being the fifth commonest cancer diagnosis, it was the third most common cancer to be treated radically with IMRT in the UK, behind breast and prostate cancer. This indicates the important role it has in HNC treatment.
In 2008, 1,237 of 7,219 patients (17.1 %) eligible for a radical course of RT received IMRT. The same study estimated that 57 % of these patients would benefit from IMRT. The relatively low utilisation of IMRT in 2008 has been addressed over the last 5 years. Improved funding and direct Department of Health guidelines that are in place to bridge the gap between the current and optimal use of IMRT have accelerated this process of IMRT implementation [45]. Such that recently updated data from 2012 show that 68 % of UK centres are now offering IMRT with 83.9 % of all UK HNC patients receiving their radical treatment using an IMRT technique [46].
Worldwide – IMRT was developed in the USA and due to the differences in health care funding has been implemented at a faster rate compared to the UK. Between 2002 and 2004, the proportion of radiation oncologists treating with IMRT was reported to increase from 40 % to 73 % [47]. As seen in the UK, the introduction of IMRT in Canada for HNC was slightly slower compared to the USA but has rapidly increased recently with 80 % of centres using IMRT for treatment of HNC in 2010 and 37 % of all centres using IMRT for “virtually all” HNC patients [48]. The reported barriers to IMRT use in Canada are now most frequently the lack of trained IMRT planners or oncologist and no longer the lack of technical capability or support staff to deliver IMRT. Other countries such as India are developing IMRT-capable facilities rapidly and recently reported that 60 of 280 centres in India are delivering IMRT treatment [49].
Potential Strategies to Improve IMRT-Induced Xerostomia
PG-sparing IMRT is now the standard treatment for HNC. However, residual xerostomia remains a clinical problem for a sizeable minority, and further improvements may be gained through avoidance of the SGs or by the further reduction in dose to the PG with the use of novel RT delivery techniques.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


