Introduction
Orthodontic tooth movement uses mechanical forces that result in inflammation in the first days. Myeloperoxidase (MPO) is an enzyme found in polymorphonuclear neutrophil (PMN) granules, and it is used to estimate the number of PMN granules in tissues. So far, MPO has not been used to study the inflammatory alterations after the application of orthodontic tooth movement forces. The aim of this study was to determine MPO activity in the gingival crevicular fluid (GCF) and saliva (whole stimulated saliva) of orthodontic patients at different time points after fixed appliance activation.
Methods
MPO was determined in the GCF and collected by means of periopaper from the saliva of 14 patients with orthodontic fixed appliances. GCF and saliva samples were collected at baseline, 2 hours, and 7 and 14 days after application of the orthodontic force.
Results
Mean MPO activity was increased in both the GCF and saliva of orthodontic patients at 2 hours after appliance activation ( P <0.02 for all comparisons). At 2 hours, PMN infiltration into the periodontal ligament from the orthodontic force probably results in the increased MPO level observed at this time point.
Conclusions
MPO might be a good marker to assess inflammation in orthodontic movement; it deserves further studies in orthodontic therapy.
Orthodontic movements promote remodeling of the alveolar bone, which is mediated by inflammatory-like reactions characterized by vascular changes and infiltration of leucocytes. During orthodontic movements, changes in the periodontium occur, depending on the magnitude, direction, and duration of the applied force. Biochemical analysis of the gingival crevicular fluid (GCF) is a useful and promising method for monitoring these changes at a single site during a certain period as well as investigating the response of dental and periodontal tissues to fixed orthodontic appliance forces. Probably, the same rationale applies to the use of whole saliva which, to a certain extent, is composed of the same substances in the GCF.
Azurophilic granules of polymorphonuclear neutrophils (PMNs) contain the enzyme myeloperoxidase (MPO), which is included in the host-derived group of compounds that are important for the tissue defense promoted by PMNs. It has been established that the level of MPO activity is directly correlated with the number of PMNs in the tissues, and, therefore, MPO activity (but not its protein amounts) is used in studies on inflammation to determine the number of PMNs. Some dental studies on the use of MPO in GCF have shown that MPO is increased in the GCF collected from inflamed sites.
Since MPO levels found in the GCF represent the extent of PMN infiltration, the determination of MPO might be a valuable tool to assess the degree of inflammation in tissues. This is particularly interesting in some processes, such as the application of orthodontic force. After the application of this force, PMNs migrate into the tissues and finally extravasate in increased numbers into the GCF.
To our knowledge, MPO activity has not yet been studied in the GCF and whole saliva of patients exposed to forces from fixed orthodontic appliances. The aim of this study was to investigate the time-dependent changes in MPO activity in whole saliva and GCF in the initial phase of orthodontic tooth movement.
Material and methods
Patients attending for routine dental treatment were invited to take part in the study. All of the patients gave written informed consent and the study had been reviewed and approved by the Institutional Review Board of the University of São Paulo in Brazil.
Fourteen orthodontic patients (5 boys, 9 girls; mean age, 12.5 ± 1.7 years) were enrolled in the study, after meeting the following criteria: good general health, no antibiotic therapy during the previous 6 months, no anti-inflammatory drug or antibiotic administration in the month preceding the study, nonsmokers, healthy periodontal tissues with generalized probing depths ≤3 mm, no radiographic evidence of periodontal bone loss, and >30 days without fixed orthodontic appliance activation.
The subjects were told not to eat or drink for 1 hour before the examination. The GCF and whole saliva were taken immediately before, and 2 hours, 7 days, and 14 days after, the fixed orthodontic appliance activation.
GCF samples were taken from the mesiobuccal or distobuccal aspects of a single-rooted tooth that received active force during the fixed appliance activation (initial alignment archwires). After isolating the tooth with a cotton roll, the crevicular site was gently dried with an air syringe. There was no supragingival plaque in the teeth from the patients of this study. The GCF samples were collected by using 1 Periopaper absorbent paper strip (Pro-Flow, Amityville, NY) placed into the sulcus or pocket until mild resistance was felt and left in place for 30 seconds. Samples were always taken from the same site at the 4 time points. Strips contaminated by saliva or blood were excluded. All strips with GCF were immediately and individually placed in Eppendorf vials containing 100 μL of buffer (50 m mol/L Tris-HCl, pH 7.4; 200 m mol/L NaCl; 10 m mol/L CaCl 2 ; and 0.02% Triton X-100). The vials were maintained on ice for 30 minutes and then centrifuged at 13,000 g for 10 minutes at 4°C. Supernatants were stored at –70°C until further analysis. All analyses were performed directly on this 100 μL sample, without further dilution and without pooling samples.
Whole saliva was collected from the patients at the same time points (baseline, 2 hours, 7 days, and 14 days). Before each collection, the patients rinsed their mouth thoroughly with water. Paraffin-stimulated whole saliva (5 mL) was collected as previously described. Briefly, the subjects chewed a piece of paraffin wax and spat the secreted saliva into a 50-mL Falcon tube. The salivary samples were immediately centrifuged at 10,000 g for 10 minutes at 4°C, and the supernatants were aliquoted and frozen at –70°C until analyses.
MPO concentrations in the GCF were measured by means of an MPO technique. Briefly, the amount of MPO in each sample was measured enzymatically by suspending the material in 2.0 mL of 0.5% hexadecyltrimethylammonium bromide (Sigma Chemical, St Louis, Mo) in 50 m mol/L potassium phosphate buffer, pH 5.4, to solubilize the MPO. After that, the MPO was assayed spectrophotometrically by addition of 1.6 m mol/L of tetramethyl benzidine (Sigma Chemical) diluted in δ-dianisidine dihydrochloride (Sigma Chemical) and 0.5 m mol/L hydrogen peroxide in a Costar 96-well plate (Corning, New York, NY). The colorimetric reading was accomplished at a wavelength of 450 nm after addition of 4 mol/L H 2 SO 4 , and the plates were read by using a μQuant microplate reader (Bio-Tek Instruments, Winooski, Vt). A standard curve was generated by using human PMNs. The PMNs were isolated from blood by density-gradient centrifugation and suspended to 1 × 10 6 cells per milliliter in PBS. MPO activity was expressed as PMNs per microliter in the GCF samples and as PMNs × 10 3 per milliliter in the whole saliva samples.
Statistical analysis
All data were analyzed for distribution; all passed the normality test and are expressed as means ± standard deviations. Statistical significances were calculated by 1-way analysis of variance (ANOVA) for multiple comparisons, followed by individual comparisons between the different time points by paired t test. Differences in the comparisons between the groups were considered significant at P <0.05.
Results
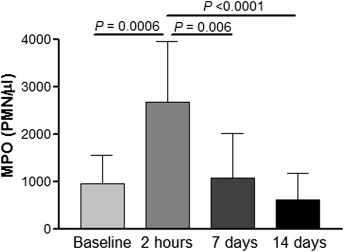
After appliance activation, increased MPO activity in the GCF was observed at 2 hours (2665 ± 1291) compared with baseline (953.3 ± 589.8, P = 0.0006), 7 days (1078 ± 923.8, P = 0.006), and 14 days (609.8 ± 561.8, P <0.0001). These results are shown in Figure 1 . The same trend was observed with MPO activity in whole saliva, shown in Figure 2 . Samples collected at 2 hours had increased activity (27.5 ± 13.3) compared with baseline (16.2 ± 3.9, P = 0.005), 7 days (17.4 ± 8.2, P = 0.02), and 14 days (13.5 ± 4.9, P = 0.002). This study showed that MPO activity is highly increased 2 hours after appliance activation, in both GCF and saliva, and that it decreases to baseline levels after 7 days. There was no statistically significant difference between MPO levels collected at 7 and 14 days, although a lower value was observed on day 14 in both saliva and GCF.