Transient and Permanent Facial Nerve Palsy
Salivary Fistula and Sialocele
Lingual or Hypoglossal Nerve Injury
Introduction
Permanent facial palsy is the most important major complication of parotid surgery. Fortunately the condition, which causes long-term disfigurement, is rare. Other minor complications such as temporary facial nerve dysfunction, salivary fistulas, disfiguring scars, painful scars, facial depression deformity secondary to the removal of gland bulk, numbness in the pinna, and gustatory sweating (Frey syndrome) are more common. Morbidities due to submandibular gland surgery, such as lingual or hypoglossal nerve injury, are comparatively rare. This chapter discusses the assessment and treatment of the most important types of morbidity associated with primary salivary gland treatment.
Transient and Permanent Facial Nerve Palsy
Definition
Facial palsy is incomplete (paresis) or complete (paralysis) peripheral palsy of the facial nerve fibers after parotid gland surgery, or much more rarely after submandibular gland surgery.
Epidemiology and Etiology
The incidence of temporary deficits after lateral and total parotidectomy varies from 18% to about 65% in the literature. When more limited approaches to the parotid gland are used, such as extracapsular dissection, the reported incidence is in the range of 10%–24%. Rates of permanent facial paresis of 2%–8% have been reported.
Diagnostic Work-Up
The severity of the palsy has to be assessed as quickly as possible, as nerve repair has to be carried out at the earliest opportunity in order to avoid degenerative changes and ensure optimal functional recovery after parotid surgery.
 The six important facial muscles covering the whole face are the occipitofrontalis, orbicularis oculi, zygomaticus major, levator anguli oris, orbicularis oris, and depressor anguli oris. All of these muscles and their functioning should be evaluated in patients with postoperative facial palsy.
The six important facial muscles covering the whole face are the occipitofrontalis, orbicularis oculi, zygomaticus major, levator anguli oris, orbicularis oris, and depressor anguli oris. All of these muscles and their functioning should be evaluated in patients with postoperative facial palsy.
A clinical examination of the most important facial functions (frowning, eye closure, nose wrinkling, lip pursing, showing the teeth, and turning down the corner of the mouth) is not sufficient. Electromyography is more accurate than a clinical examination. Voluntary electromyographic activity can often be observed in some of the facial expression muscles even when the clinical examination has shown complete paralysis. In this case, the palsy is defined as incomplete. Single-fiber activity or a zero line during voluntary electromyographic activity indicates complete palsy of the nerve branch target area being examined.1
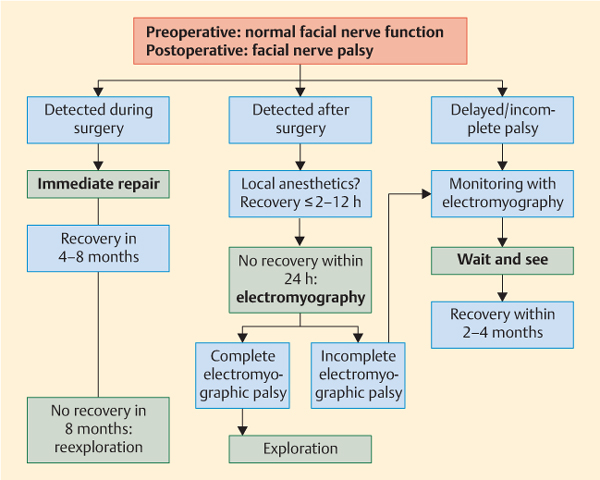
Distinguishing between incomplete and complete palsy is very important for the subsequent treatment plan (Fig. 39.1). When an effect of local anesthetic administration can be ruled out, any complete electromyographic palsy requires surgical exploration of the nerve. Magnetic resonance imaging and computed tomography are normally not helpful, as there are too many postoperative artifacts. Patients with incomplete electromyographic palsy have to be followed up until facial nerve recovery is achieved. As in patients with delayed palsy—i.e., an onset of palsy no earlier than 48 hours after surgery—surgical reexploration does not provide better functional results than a wait-and-see strategy. Pathological spontaneous muscle activity (pathological sharp waves and fibrillation potentials) does not develop earlier than 14 days after the onset of the injury. It represents evidence of a degenerative lesion, but is only helpful in patients with a delayed presentation after surgery.
Fig. 39.1 Algorithm for the management of facial palsy after parotid surgery.
Therapy
The techniques for facial nerve repair and adjuvant treatment are described in Chapter 20. If a wait-and-see strategy is being used in patients with incomplete palsy, additional drug treatment—e.g., with corticosteroids—is not recommended, as it has not been shown that this offers any functional benefit.
Salivary Fistula and Sialocele
Definition
A sialocele is an accumulation of saliva without drainage to the skin, either inside a main salivary duct due to postoperative closure of the duct—i.e., a retention cyst—or an accumulation within the residual gland due to disruption of the main salivary duct branches inside it. An accumulation inside the gland leads to a local inflammatory reaction, which forms a pseudocapsule around the sialocele. If sustained saliva production leads to sialorrhea from the wound, the condition is known as salivary fistula. A collection of fluid from a salivary fistula under the skin flap may mimic a sialocele.
Epidemiology and Etiology
The incidence of transient salivary fistula or sialocele formation is less than 5%. Fewer than 1% of patients have chronic problems.
Diagnostic Work-Up
A biochemical analysis of the wound or fistula fluid for amylase confirms that it is saliva. Magnetic resonance sialography is the best imaging tool for visualizing the salivary duct system and is helpful for localizing or ruling out a sialocele. In special cases in which it is difficult to locate the fistula exit, it may be helpful to inject methylene blue into the natural ostium of the salivary duct—e.g., when there is a parotidoantral fistula to the maxillary sinus.
Therapy
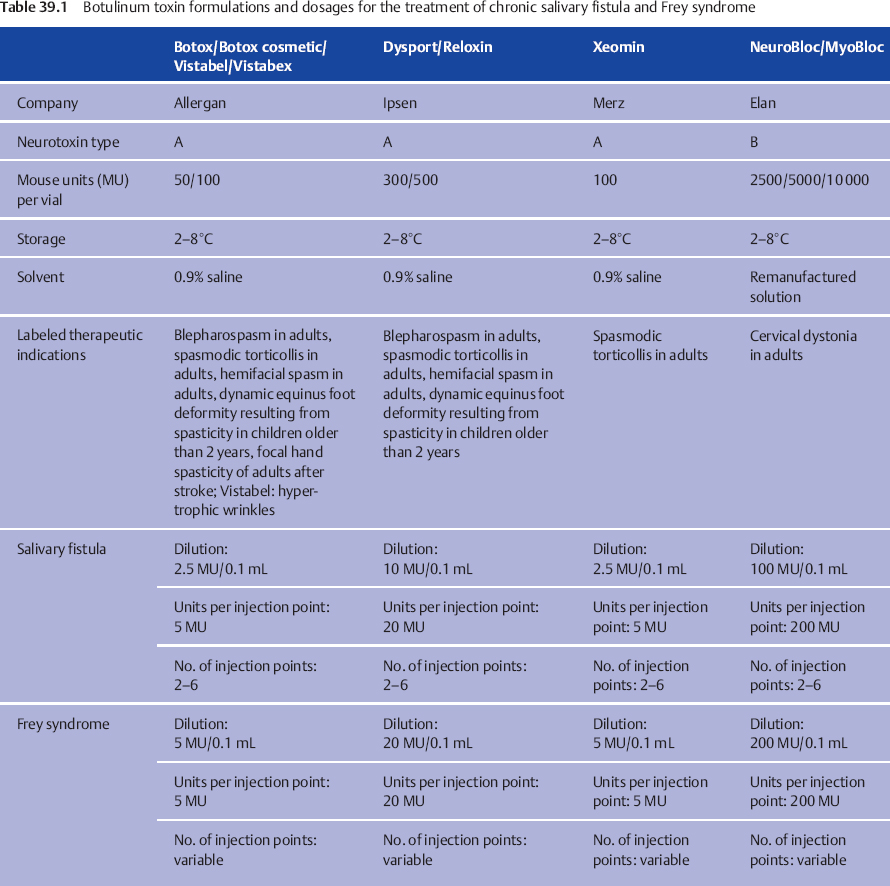
Salivary fistulas can in most cases be managed conservatively with repeated aspiration and compression. Superinfection, mainly by Staphylococcus aureus, requires antibiotic treatment. The fistulas resolve within 4–6 weeks. Chronic fistulas due to major duct injuries are more resistant to conservative treatment. In these cases, the treatment of choice is single or repeated botulinum toxin injections into the residual gland. As in botulinum toxin treatment in children with drooling (see Chapter 12), the aim is to stop saliva production temporarily. Details of the available formulations, concentrations, and dosages are given in Table 39.1. In comparison with the treatment of Frey syndrome (see below), lower concentrations but larger volumes are needed to reach the whole gland. Depending on the size of the gland remnant, 4–10 different points in the parotid gland are infiltrated with botulinum toxin. It is strongly recommended that the injections should be performed with ultrasound guidance using a 7.5-Hz linear transducer. Ultrasound makes it easy to control the distribution of the botulinum toxin in the salivary gland and avoid injection into the surrounding tissue. The effects start after 7–14 days and persist for 6–8 weeks, depending on the dosage.
If botulinum toxin treatment is contraindicated or fails, other options, such as long-term pressure dressings, tympanic neurectomy, tissue graft interpositioning, or surgical closure of the salivary duct should be considered. The efficacy of these strategies is doubtful and they may require several months. Revision surgery is often a last resort, but this is risky for the facial nerve due to local inflammation of the wound in the gland remnant, leading to extensive scar formation.
Gustatory Sweating
Definition
The pathophysiological explanation for Frey syndrome (Fig. 39.2) is aberrant regeneration of the parasympathetic cholinergic fibers that normally innervate the parotid gland. Having lost their target after parotidectomy, the parasympathetic fibers regenerate into local facial skin vessels and sweat glands. Activation of these aberrant fibers during meals results in local skin vasodilation (gustatory flushing) and localized sweating (gustatory sweating). Typically, the patient presents about 6–12 months after parotidectomy and reports disturbing localized sweating in the face, ear region, or temporal hairline on the operated side.
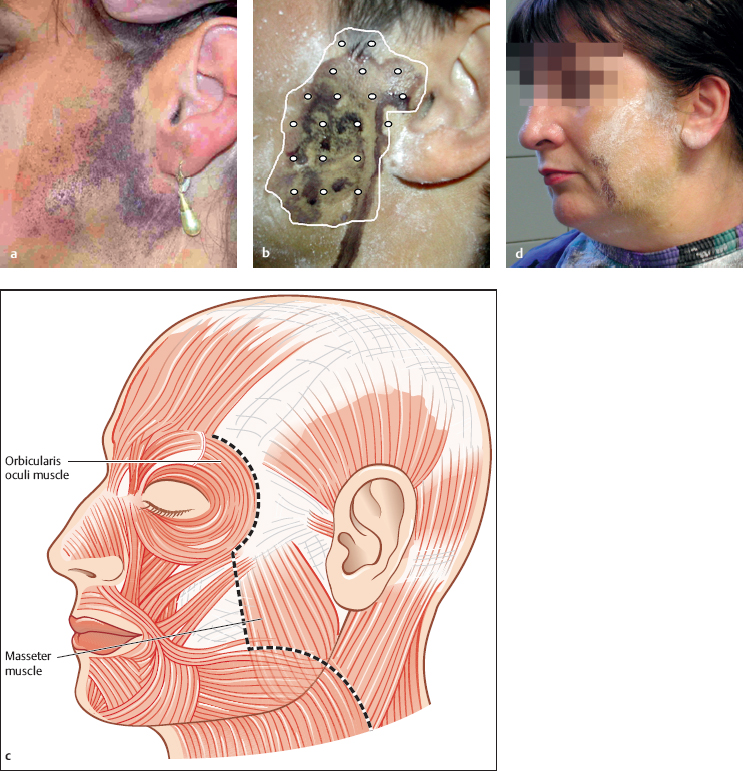
Fig. 39.2 a–d Botulinum toxin injection for Frey syndrome.
a The Minor iodine starch test has been used to outline the area.
b The botulinum toxin is injected with a distance of 1 cm between the injection points.
c Care should be taken not to inject anterior to the anterior border of the sternocleidomastoid muscle, to avoid unintended facial nerve weakness.
d Observing the above rule can lead to an untreated area being left, but this is without functional relevance, as a check-up with a Minor test 4 weeks after treatment shows.
Epidemiology and Etiology
Gustatory sweating, or Frey syndrome, is an unavoidable sequela of parotid surgery. On the basis of results with the Minor iodine starch test, the incidence after parotid gland surgery has been reported to be up to 100%. Half of the patients have gustatory sweating symptoms, and approximately 15% consider the symptoms severe and require treatment.2
Diagnostic Work-Up
The Minor iodine starch test is the best method of locating the skin region involved in gustatory sweating (Fig. 39.2a). The patient’s face and the hair region on the affected side are painted with an iodine solution, which is allowed to dry for 1–2 minutes. The whole area is then dusted with starch powder. The patient starts eating a sialogogue (e.g., a lemon slice or an apple) to evoke gustatory stimulation. Within 5 minutes, the starch turns blue in the area in which moisture is produced.
 An iodine tincture (15 g iodine, 100 mL castor oil, 900 mL ethanol) and starch powder are all that is needed for the Minor iodine starch test.
An iodine tincture (15 g iodine, 100 mL castor oil, 900 mL ethanol) and starch powder are all that is needed for the Minor iodine starch test.
< div class='tao-gold-member'>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses