Introduction
The purpose of this randomized clinical study was to evaluate the presence of the periodontal pathogen Aggregatibacter actinomycetemcomitans on metallic brackets and the effectiveness of a 0.12% chlorhexidine digluconate mouthwash in inhibiting this microorganism.
Methods
The study involved 35 patients of both sexes having orthodontic treatment with fixed appliances between the ages of 14 and 22 years, randomized into 2 groups: experimental (n = 17) and control (n = 18). Two new metallic brackets were placed on the patients’ premolars, and the subjects rinsed with a solution of 0.12% chlorhexidine digluconate or a placebo solution twice a week for 30 days. After that, the brackets were removed and underwent microbiologic analysis with the checkerboard DNA-DNA hybridization technique. Data were analyzed by using the Student t , Fisher exact, and Mann-Whitney tests at the significance level of 5%.
Results
The results showed that A actinomycetemcomitans was present in all brackets from the subjects in the control group vs 83% of the subjects who rinsed with chlorhexidine digluconate ( P <0.0001). There were also significantly lower levels of this species in the chlorhexidine digluconate group compared with the control group ( P = 0.0003).
Conclusions
We concluded that 0.12% chlorhexidine digluconate rinsing, twice a week for 30 days during orthodontic treatment, is effective in reducing the presence and levels of A actinomycetemcomitans on metallic brackets.
Orthodontic appliances create a favorable environment for the accumulation of microbial biofilm, which can lead to adverse effects on the periodontium. The periodontal pathogen, Aggregatibacter actinomycetemcomitans , is a gram-negative cocobacillus, considered a main pathogen of inflammatory periodontal disease, and has been related to aggressive periodontitis. It is also associated with certain systemic infections such as endocarditis, meningitis, osteomyelitis, and brain abscesses.
Periodontitis is difficult to control by mechanical removal of the subgingival biofilm alone, and studies have indicated that this kind of treatment is not effective in eliminating A actinomycetemcomitans . Therefore, there is great interest about the effects of systemic antibiotics and other antimicrobial agents in controlling this periodontal pathogen. Several commercially available mouthwashes are used to improve oral hygiene, although their efficacy is controversial. To improve the knowledge on this matter, antimicrobial studies have been conducted; however, most of them were tested against cariogenic bacteria. Chlorhexidine is a topical antimicrobial agent that has positive effects in inhibiting plaque formation and reducing the bacteria in the oral cavity.
Microbial diagnostic tests with genomic DNA probes have been widely used in the detection and quantification of pathogenic microorganisms, because they are faster and more reliable than conventional microbiologic culture techniques. The checkerboard DNA-DNA hybridization technique allows rapid identification of several bacterial species in a large number of oral samples. This technique has been used in the dental areas of periodontology, endodontics, implantology, pediatric dentistry, and cariology. In orthodontics, this technique was used to compare the total bacterial counts on ceramic and metallic brackets, and it has been shown that metallic brackets can be highly contaminated with A actinomycetemcomitans .
Therefore, the aim of this pilot clinical study was to investigate, in vivo, the contamination of metallic brackets by the periodontal pathogen A actinomycetemcomitans by using the checkerboard DNA-DNA hybridization technique and the efficacy of a 0.12% chlorhexidine mouthwash in controlling this microorganism.
Material and methods
Eligible participants were selected from patients of both sexes in orthodontic corrective treatment for less than 16 months at the orthodontic clinic of the School of Dentistry of Ribeirão Preto, University of São Paulo, in Brazil. They had good general health and had not used antibiotics or antimicrobial mouthwashes within 3 months before the study. Thirty-five 14- to 22-year-old patients who met these inclusion criteria were enrolled as participants. The research protocol was reviewed and approved by the institutional research ethics committee (process 2008.1.163.58.8).
One week before the beginning of the study, each patient’s plaque index was determined by 1 operator (M.C.D.A.) according to the method of Silness and Loe to confirm that all patients had similar amounts of dental biofilm. A biofilm disclosing agent was used, and scores were attributed to the 4 sides of each tooth as follows: 0, no biofilm on tooth surface; 1, biofilm was invisible to the naked eye but could be collected with a probe; 2, biofilm was visible at the gingival margin; and 3, biofilm was visible at the gingival margin and covered a significant portion of the tooth surface. The average score for a tooth was equal to the sum of the scores for all sides divided by 4, and the plaque index for each patient was equal to the sum of the average scores for all teeth divided by the number of teeth. Next, dental biofilm deposits were cleaned by using nonfluoridated pumice and water. The patients were instructed to brush their teeth at least 3 times a day after meals using a toothbrush (Professional; Colgate-Palmolive Indústria e Comércio, São Paulo, São Paulo, Brazil) and fluoride-containing dentifrice (Máxima Proteção Anticáries; Colgate-Palmolive Indústria e Comércio) supplied by the researchers throughout the experimental period. In this study, all patients had similar baseline levels of dental plaque (range, 0.5-1.5) and were periodontally healthy.
The 35 patients were randomized to 2 groups (experimental and control) by using statistical software (version 9.1.3 for Windows; SAS Institute, Cary, NC). In all patients, 2 new sterile edgewise metallic orthodontic brackets (0.022 × 0.028-in slot) (Generus; GAC International, Bohemia, NY) were bonded with orthodontic light-cured composite (Transbond XT; 3M Unitek, Monrovia, Calif) to 2 premolars (maxillary right or left and mandibular right or left) selected randomly with the SAS software. Seventeen patients used 0.12% chlorhexidine mouthwash (Periogard; Kollynos do Brasil, Osasco, São Paulo, Brazil) (experimental) as an antimicrobial agent, and 18 patients used a placebo mouthwash (Erva Doce Pharmacy, Ribeirão Preto, São Paulo, Brazil) with color, taste, and composition similar to those of Periogard (control). In both groups of patients, oral rinses were done with 10 mL of the test solution for 30 seconds twice a week (Tuesdays and Fridays) for 30 days. On Tuesdays, mouth rinsing was performed at the dental school under the researchers’ supervision; on Fridays, mouth rinsing was done at the patients’ homes at night, 1 hour after toothbrushing. Both solutions were stored in 120-mL plastic bottles and were given to the subjects every week after prophylaxis. The patients were blinded to which mouthwash was being used.
After 30 days, the brackets were removed with pliers (How 110; 3M Unitek) by a trained, calibrated operator (M.C.D.A.) (orthodontist) in a blinded fashion and sent for analysis. Two brackets from each patient of both groups were placed in individual labeled plastic tubes (Eppendorf AG Barkhausenweg, Hamburg, Germany), containing 150 μL of Tris-EDTA buffer solution (pH 7.6) and 100 μL of 0.5 M sodium hydroxide, and were agitated for microbial desorption. Next, the brackets were removed with sterile clinical pliers, and the plastic tubes containing the bacterial suspension were stored frozen at –20°C for further analysis with the checkerboard DNA-DNA hybridization technique. The patients received new brackets, and corrective orthodontic treatment proceeded as planned.
The DNA probe was prepared by using whole genomic DNA from A actinomycetemcomitans (strains 43718 and 29523). The collected samples were boiled for 10 minutes to produce cell lysis and DNA denaturation. The DNA was then fixed in individual lanes on a positively charged nylon membrane (Boehringer Mannheim, Indianapolis, Ind) by using a checkerboard slot blot device (Minislot 30; Immunetics, Cambridge, Mass). The digoxigenin-labeled genomic DNA probe (Roche Applied Science, Indianapolis, Ind) was then hybridized perpendicularly to the lanes of the clinical samples by using a Miniblotter 45 apparatus (Immunetics). Bound probes were detected with phosphatase-conjugated antibody to digoxigenin (Roche Applied Science). After incubation in a solution containing the CDP-Star substratum (Amersham Pharmacia Biotech Inc, Piscataway, NJ), the membranes were placed in an autoradiography cassette under a radiographic film (X-Omat; Kodak, Rochester, NY), developed for chemiluminescence signal detection. Signals were evaluated visually by comparing with the standards at 10 5 and 10 6 bacterial cells of the test species on the last 2 lanes of the same membrane. This provided the approximate number of bacterial cells per sample for the bacterial strain evaluated; this was equal to the sum of the values obtained in the 2 brackets removed from each patient. The data were read twice by a blinded examiner (M.C.D.A.) (kappa, >0.8). The sensitivity of this assay was settled to allow detection of 10 4 cells. To permit semiquantitative examination of chemiluminescence signals for A actinomycetemcomitans in each sample, the intensity of the signals (ie, contamination of the brackets) was evaluated at the following levels: 0 (not detected), 1 × 10 4 , 1 × 10 5 , 5 × 10 5 , 1 × 10 6 , and 1 × 10 7 . Data were analyzed by using the Student t , Fisher exact, and Mann-Whitney tests at the significance level of 5%.
Results
The 35 patients selected participated for the entire study period. The results of the randomization analysis of the subjects in the control and experimental groups are described in Table I . There was no statistical difference in the sex of the members of the 2 groups ( P >0.05). However, the subjects in the experimental group had a higher average age than did those in the control group ( P = 0.02).
| Control group (n = 18) | Experimental group (n = 17) | P | |
|---|---|---|---|
| Age (y) | 15.3 ± 1.3 | 20.6 ± 1.7 | 0.02 ∗ |
| Sex | |||
| Female | 44.5% | 58.8% | 0.06 † |
| Male | 55.5% | 41.2% | |
| Plaque index | |||
| Baseline | 0.92 ± 0.30 | 1.04 ± 0.24 | 0.19 ∗ |
| 30 days | 0.88 ± 0.37 | 0.51 ± 0.15 | 0.0006 ∗ |
| P | 0.2358 ‡ | < 0.0001 ‡ |
∗ P value for the Student t test.
† P value for the Fisher exact test.
‡ P value for the paired Student t test, comparing baseline vs 30 days.
At baseline, all patients had similar levels of dental plaque ( P = 0.19), whereas after using the mouthwash with chlorhexidene, the experimental group had less dental plaque accumulation compared with the control group ( P = 0.0006) ( Table I ).
In terms of prevalence, A actinomycetemcomitans were detected in 100% of the brackets in the control group and in 82.3% of the brackets in the experimental group ( P <0.0001). In addition, it was observed that almost 40% of the brackets in the control group had higher levels of A actinomycetemcomitans (5 × 10 5 ) vs less than 10% in the chlorhexidine group. Conversely, about 5% of the brackets in the control group and more than 50% of those in the experimental group had low levels of this species (1 × 10 4 ) ( Fig ).
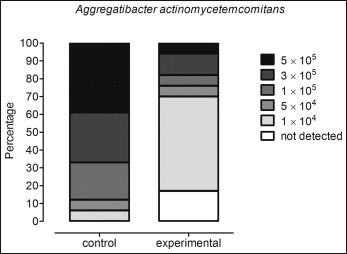
When the total levels were considered, the experimental group, in which the patients used the chlorhexidine mouthwash, showed a significantly lower level of this pathogen compared with the controls ( P = 0.0003) ( Table II ).
| Control group (n = 18) | Experimental group (n = 17) | P | |
|---|---|---|---|
| A actinomycetemcomitans | 300,000 (100,000-500,000) | 10,000 (5,000-77,500) | 0.0003 ∗ |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


