Fig. 11.1
Histological sample of necrotic bone of an MRONJ patient with intravenous bisphosphonates. Areas with bacteria surrounded by acute inflammatory infiltrate (black arrows). In the middle are the typical signs of Actinomyces (gray arrow) colonies (H&E stain, 100×)
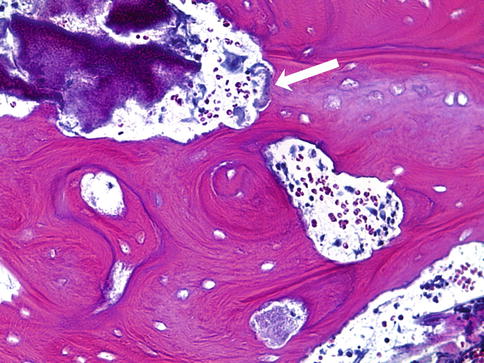
Fig. 11.2
Histological sample of an MRONJ patient. Close to the bony surface, a biofilm is visible (arrow). Necrotic bone visible with empty osteocytic lacunae (H&E stain; 400×)
Biofilms are usually defined as surface-associated microbial communities, surrounded by an extracellular matrix of polymeric substances. Biofilm bacteria demonstrate coordinated behavior such as the formation of complex three-dimensional structures and functionally heterogeneous bacterial communities. The biofilm formation itself is an important microbial survival strategy [8, 14]. The bacterial size in biofilms ranges from 0.5 to 10 μm [8]. They are able to persist in a stationary phase-like dormancy within the biofilm, which may be responsible for their general resistance to antibiotics. Even if the matrix may not inhibit the penetration of antibiotics into the biofilm completely, it may reduce the rate of penetration enough to induce the expression of genes within the biofilm to mediate resistance. The (electrostatic) charge of polymers and the antibiotic-degrading enzymes in the matrix may lead to reduced binding and deactivation. Even dead cells may dilute antibiotics. Another mechanism of tolerance is an efflux pump expression in biofilms as demonstrated for tobramycin, gentamicin, and ciprofloxacin resistance [14].
It has also been reported that bisphosphonates enhance bacterial adhesion to bone hydroxyapatite. Kos et al. demonstrated an increased bacterial adhesion in the presence of pamidronate on hydroxyapatite. They tested Staphylococcus aureus and Pseudomonas aeruginosa strains. These observations emphasize the infectious aspect of osteonecrosis of the jaw, once again [15]. Ganguli et al. found significantly higher adherence of bacteria on hydroxyapatite-coated ceramic artificial hip joints, if these were coated with pamidronate when compared to clodronate or control [16]. This suggests that bacterial adhesion to bone coated with bisphosphonates may be mediated by proteins termed “microbial surface components” which recognize adhesive matrix molecules. The amino-terminal domain binds by direct electrostatic interactions through a direct surface protein interaction or by providing an amino acid on the surface of the bony hydroxyapatite, which interacts with these molecules and mediates increased bacterial adhesion [17]. In vitro and in vivo experiments showed that a combination of pamidronate and Fusobacterium nucleatum caused the death of gingival fibroblasts and the downregulation of growth factors of keratinocytes, responsible for epithelial cell growth and migration [18].
Regularly Observed Microbiological Findings
Though oral infection is a common finding, the therapist should know the special characteristics of the oral cavity and its local microflora. A distinct number of aerobic and anaerobic species are regularly observed and described. The bacteria identified in the bone specimens comprised Gram-positive and Gram-negative organisms. These also include aerobes, although anaerobes and facultative anaerobes dominate [8]. Anaerobic species are from the Peptostreptococcus, Prevotella, Fusobacterium, Gemella, and Porphyromonas genera. Aerobic species are Streptococcus, Staphylococcus, and Corynebacterium. Each of these microorganisms occupies a different microniche, but the prevailing balance is easily disturbed. Especially pathogenic or opportunistic bacteria (Actinomyces, Prevotella intermedia), yeasts (Candida sp. Histoplasma capsulatum), viruses, and parasites profit from any disturbance of the equilibrium and can then harm the organism [10]. Denaturing gradient gel electrophoresis profile and dice coefficient described 3 predominant genera in MRONJ, namely, Streptococcus, Eubacterium, and Pseudoramibacter [3].
The biofilms in these patients have a distinct combination of species/phylotypes, segregating a population with compositional changes but maintaining functional similarity of acid production [2]. The development of biofilms on the surface of the exposed bone may account for the poor response to systemic antimicrobial therapy as described above [19]. Bone specimens from MRONJ-affected sites showed large areas of occluded biofilms comprising mainly bacteria, and occasionally yeast, embedded in extracellular polymeric substance. The number of bacteria in these biofilms ranged from 2 to 15 and included species from genera Fusobacterium, Bacillus, Actinomyces, Staphylococcus, Streptococcus, and Selenomonas and 3 different types of treponemes [8]. The phyla described by Ji et al. found in MRONJ lesions are Acinetobacter, Bacteroidetes, Chloroflexi, Cyanobacteria, Firmicutes, Fusobacteria, Proteobacteria, and Synergistetes [2]. Badros et al. identified Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Streptococcus sp., and Eikenella species [20]. MRONJ site biofilm contained predominantly Fusobacterium, Actinomyces, Staphylococcus, Streptococcus, Selenomonas, and Treponema species as reported by Sedghizadeh and coworkers [8]. These different research teams demonstrated similarities but also differences in their findings. Though principally microbial cultures from the areas of infected exposed bone will show normal oral microbes, it must be taken into consideration that in cases with extensive soft-tissue involvement, microbial culture data may facilitate the selection of an appropriate antibiotic [19]. Awareness, however, must be drawn to the length of time the bone was exposed to the oral cavity as nonpermanent bacteria species may infect the bone as the nature of the biofilm is expected to change and adapt within time [8]. Interestingly, some histopathological examinations have even indicated that edentulous jaws contain regions of necrotic bone and microbial biofilm formation even after one year of tooth extraction and mucosal healing resulting eventually in MRONJ formation [21].
Though microbiology findings in MRONJ bone are dependent on the local oral flora, the spectrum may vary considerably. Some authors do not recommend microbial testing in principal, but nevertheless, it is the prerequisite for an adequate antibiotic regime and must be therefore recommended.
Actinomyces
Actinomyces species are frequently observed in MRONJ. Actinomyces is regularly observed in histological bone specimens (Fig. 11.1) of MRONJ patients. In 70 to 100 % of all cases, Actinomyces is present [22–24]. In the beginning of the MRONJ discussion, Actinomyces was considered to be a particular complication [24] and to be an underestimated agent in the pathogenesis of MRONJ [11].
Actinomyces is a non-spore-forming, anaerobic, or microaerophilic bacterial species of the genus Actinomyces. Actinomyces spp. are Gram-positive, pleomorphic, and commonly delicately filamentous microbes. Actinomyces can be commonly found in gingivodental crevices [11, 24] and must be suspected as an opportunistic infection [10, 24, 25]. Mucosal disruption is also the key step in pathogenesis. Histologically Actinomyces can form clumps called sulfur granules [11]. Even though Actinomyces spp. were frequently found in exposed necrotic bone, however, the development of MRONJ also occurs in its absence [22, 24].
Candida, Fungal Infection, and Principal Considerations
Infections with Candida spp. are also described regularly. Infections with other fungal spores and hyphae have been observed also frequently (Fig. 11.3) [24], but yeasts that have been identified consistently included Candida spp. [8, 10, 26]. Analysis of oral lesions demonstrated a correlation between higher Candida colonization and the severity of the lesion [27].
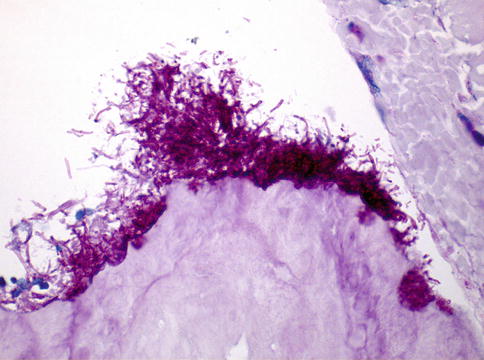

Fig. 11.3
Fungal spores and hyphae adjacent to the bone (PAS stain, 400×)
It must be taken into consideration that most of the addressed bacteria are strictly dependent on the culture-dependent methods used. Though most of the infectious diseases of the oral and maxillofacial regions are caused by polymicrobial infections of anaerobes and all causative bacteria may not be cultivable by the method used, they cannot be excluded or denied. Therefore, critical analysis of the identified bacteria and yeasts must be compared with the clinical success by antibiotic and antiyeast therapy. An antibiotic regime that does not support the microbiology findings could show a good clinical success and vice versa. Therefore, the clinical outcome is the leading sign for the antibiotic regime.
Antibiotic Therapy
Early in 2005, Bamias et al. described that treatment with antibiotics resulted in transient improvement of MRONJ after multiple courses of antibiotics. It is possible to improve the infectious symptoms in the majority of cases with antibiotics. It is also evident in most cases that the purulent discharge and pain recurred after discontinuation of the antibiotics [28]. The time till the relapse and worsening of the infectious symptoms may vary.
Antibiotics Recommended
The “best antibiotic of choice” should be bactericidal, have no side effects, and be economic. The antibiotic should include the resident bacteria but also cope to the considered changed bacteria by the disease and time of exposure.
Recommended antibiotics include penicillin, amoxicillin, metronidazole, quinolones, clindamycin, doxycycline, erythromycin, and ciprofloxacin [2, 4, 6]. The German S3 guideline addresses amoxicillin and clindamycin [25].
Analysis of 391 cases reported in literature demonstrated that most patients were given a combination of antibiotics, either in association or iteratively. Beta-lactam antibiotics were prescribed most frequently. These antibiotics included aminopenicillins (39 %), aminopenicillins with beta-lactamase inhibitor (28 %), methoxyphenyl penicillin (26 %), clindamycin (33 %), metronidazole (13 %), tetracyclines (11 %), and fluoroquinolones (1 %).
The sensitivity of bacteria located in the oral cavity to antimicrobials however is on the decline with a marked trend towards resistance. Prevotella and Porphyromonas are genera resistant frequently to a higher number of antibiotics and subsequently 80 % sensitive to amoxicillin plus clavulanate, clindamycin, and metronidazole. Oral streptococci and Eikenella corrodens have a resistance to special antimicrobial agents, particularly macrolides (35–70 %) and to penicillin and clindamycin (10–15 %). Resistance to metronidazole, macrolides, and first- and second-generation cephalosporins is also described. In contrast, Eikenella corrodens is sensitive to amoxicillin [10].
Surgery should always be supported by an antibiotic regime, as recommended by the German S3 guideline [25]. This antibiotic regime is generally advised as prophylactic support in elective surgery (e.g., extraction), surgery of MRONJ, or conservative treatment of MRONJ. The time recommended for therapy noticeably varies in literature.
Eckert and coworkers recommend ampicillin plus clavulanic acid 7 days postoperatively [29], and Bagan et al. advised a minimum of 10 to 30 days [30]. In case of allergies, clindamycin is recommended as an alternative [30]. Penicillin and metronidazole given intermittently or continuously for 1 week intravenously and then orally for the next 3 weeks were also described [31, 32]. Therapy with amoxicillin plus clavulanic acid for 10 days followed by 3 weeks of doxycycline has also been recommended [33]. Stanton et al. prescribed levofloxacin before surgery and in combination with metronidazole for 4–6 weeks postoperatively [34].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


