Chapter 63 Management of sleep-related breathing disorders in children
1 INTRODUCTION
Sleep-related breathing disorders (SRBD) are a common occurrence in children. While such events may be central in etiology, most affected children experience obstructive sleep disorders, caused by anatomic variations and complicated by the diminished pharyngeal muscle tone and neurophysiologic changes that typically accompany sleep. Hyperplasia of the tonsils and adenoid is the most common cause of such obstruction, but infants and toddlers may be affected at a variety of sites in the upper respiratory tract. Causes of SRBD in children may be grouped on the basis of age, simplifying the differential diagnosis for a given patient (Table 63.1). In small children, caliber of the airway may be reduced due to nasal obstruction, compromised skeletal anatomy, hyperplastic pharyngeal or laryngeal soft tissues or neuromuscular compromise. In addition, in older children, obstruction due to obesity has been encountered with increasing frequency over the last two decades.1,2
Table 63.1 Causes of sleep-related breathing disorders in children
| Neonates and infants |
| Nasal aplasia, stenosis, or atresia |
| Nasal or nasopharyngeal masses |
| Craniofacial anomalies: |
| Hypoplastic mandible (Pierre Robin, Nager’s, Treacher Collins) |
| Hypoplastic maxilla (Apert’s, Crouzon’s) |
| Macroglossia (Beckwith–Wiedemann) |
| Vascular malformations of tongue and pharynx |
| Congenital cysts of the vallecula and tongue |
| Neuromuscular disorders |
| Toddlers and older children |
| Rhinitis, nasal polyposis, septal deviation |
| Syndromic narrowing of nasopharynx (Hunter’s, Hurler’s, Down’s, achondroplasia) |
| Adenotonsillar hyperplasia |
| Obesity |
| Macroglossia (Down’s) |
| Vascular malformations of tongue and pharynx |
| Neuromuscular disorders |
| Iatrogenic |
| Nasopharyngeal stenosis |
2 DIAGNOSIS OF SRBD IN CHILDREN
In most children with SRBD, the presenting symptom is stertor, a term used to describe sonorous breathing in the upper airways, including snoring. The prevalence of snoring during sleep has been reported as 3–12% in children,3 although some studies suggest the rate may be as high as 27%.4 Primary snoring is generally not considered a form of SRBD, although some studies do suggest the possibility of associated morbidity such as elevated blood pressure and reduced arterial distensibility.5
In its mildest form, SRBD presents as upper airway resistance syndrome (UARS). Affected children demonstrate episodic arousals resulting from partial obstruction of the upper airway, associated with symptoms of heroic snoring, mouth-breathing, sleep pauses or breath-holding, gasping, perspiration, and enuresis. Daytime manifestations of sleep disturbance include morning headache, dry mouth, halitosis, and most significantly behavioral and neurocognitive disorders.6 Hypersomnolence may occur in older children and adolescents. Other signs and symptoms include audible breathing with open mouth posture, hyponasal speech, and chronic nasal obstruction with or without rhinorrhea. Approximately 40% of children who snore demonstrate more significant degrees of obstruction characteristic of obstructive hypopnea syndrome (OHS) or obstructive sleep apnea syndrome (OSAS) as defined below.6 The most severely affected patients may develop cor pulmonale, right ventricular hypertrophy, congestive heart failure, alveolar hypoventilation, pulmonary hypertension, pulmonary edema, or failure to thrive, and are at risk for permanent neurological damage and even death.
When a history of severe symptoms of sleep disturbance correlates with obvious physical findings of airway obstruction, additional studies to establish a patient’s candidacy for surgical intervention may be superfluous. However, studies suggest that in most cases accurate diagnosis of SRBD cannot be established solely on the basis of a history and physical examination.7 SRBD occurs primarily during REM sleep when children are less likely to be observed by their parents,8 and in many cases of UARS and OHS parents may misinterpret the symptoms only as snoring in the absence of obstruction. In addition, airway dynamics during sleep cannot be determined by static examination in the office setting, and fiberoptic assessment of the airway is often deceptive due to the distorted wide-angle view. Similarly, radiographic assessment of the adenoid tissue and tongue base may be difficult to interpret. In such cases, polysomnography (PSG) remains the gold standard for objective correlation of ventilatory abnormalities with sleep disordered breathing.7 However, the severity of obstruction for which intervention is recommended remains controversial, with most authors advocating aggressive management of children whose Respiratory Distress Index (RDI; obstructive apneas+hypopneas per hour) is between 1 and 5. Furthermore, the expense and scheduling difficulties associated with PSG make this a cumbersome method of assessment in many otolaryngology practices. Other techniques of assessment including audiotaping, videotaping, and home and abbreviated PSG have demonstrated favorable results, but require further study.
3 MANAGEMENT OF SRBD IN CHILDREN
3.1 NON-SURGICAL MANAGEMENT
Treatment of SRBD in children is tailored to the etiology of the airway obstruction. Medical management, such as thioxanthines and methylphenidate, may be useful in cases of central apnea. Pharmacotherapy may also be considered in less severe cases of obstructive apnea, or when surgical intervention does not address the pathology. Examples of such disorders include neonatal rhinitis, allergic rhinitis, and acute tonsillitis. Systemic steroids are effective in reducing lymphoid hyperplasia, but the results are often transient. In cases of chronic upper airway obstruction, positive airway pressure has demonstrated high efficacy in treating SRBD in children; however, the dropout rate is high and the rate and duration of nightly use are relatively low even among compliant children.9 Other alternatives, such as mechanical correction by prostheses, orthodontia, or weight loss, may be worth consideration as well, although these interventions are rarely tolerated in children and are often ineffective. As a result, even in cases of SRBD due to obesity and neuromuscular impairment, surgical management is generally considered as first-line therapy and other modalities are entertained postoperatively to address residual obstruction.
3.2 SURGICAL MANAGEMENT
3.2.1 PREOPERATIVE PLANNING
Preoperative planning is an essential component of the surgical management of patients with SRBD. Such individuals are prone to airway embarrassment during induction of anesthesia, and the surgeon and anesthetist should discuss emergency intervention for the airway should the patient become acutely obstructed. In most cases, dynamic airway collapse may be overcome by bag/mask ventilation used with an oral airway. In patients with severe micrognathia, macroglossia, or lingual tonsil hyperplasia, visualization of the larynx may be compromised. In such cases, the operating team should preoperatively assemble those items necessary for intubation with a Bullard laryngoscope, flexible fiberoptic bronchoscope, or fiberoptic intubation wand, and patients should be intubated under conditions of maximal topical and minimal systemic anesthesia, preferably in the sitting position.
Postoperative respiratory distress is common after surgery for SRBD due to effects of anesthesia, bleeding, edema, and residual airway compromise. Patients at greatest risk include those with severe OSAS, diminished neuromuscular tone (i.e. cerebral palsy), morbid obesity, skeletal and craniofacial abnormalities such as hypoplasia of the midface and/or mandible or nasopharyngeal vault, and very young children (under age 2–3).10,11 As a result, high-risk individuals who are undergoing even routine procedures such as adenotonsillectomy should be admitted to a high-visibility bed in the hospital with continuous cardiac and oxygen saturation monitoring. Intraoperative use of steroids and postoperative placement of nasopharyngeal airways and tongue sutures for anterior traction may reduce the risk of airway compromise after surgery. Narcotics and sedatives should be used sparingly in severely obstructed children. Obese patients and those with reduced neuromuscular tone may benefit from airway support with positive airway pressure. In the most extreme cases, overnight endotracheal intubation may be desirable. If it is suspected that the airway may be difficult to reintubate should obstruction occur after extubation, the tube should be remove in the operating room setting.
3.2.2 NASAL AND NASOPHARYNGEAL OBSTRUCTION
While choanal atresia may be approached by either the transpalatal or the transnasal route, improvements in endoscopic and powered instrumentation have made the transnasal approach the first choice for most otolaryngologists. Our preferred approach is as follows.
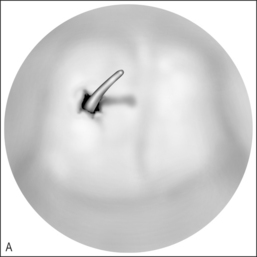
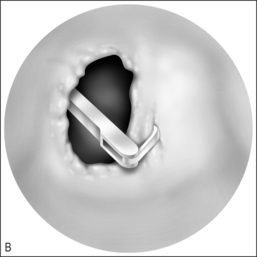
In cases of piriform aperture stenosis, the offending bone may be approached through a sublabial approach (Fig. 63.2), and the aperture is widened using the 2.9 mm round burr at high speed. Stents may be placed as in choanal atresia repair if desired.
Restenosis is the most common complication of these procedures. Success rates (permanent patency) in recent studies are quoted as 78% to 95% with 1–3 year follow-up; number of required revisions is very variable.12,13
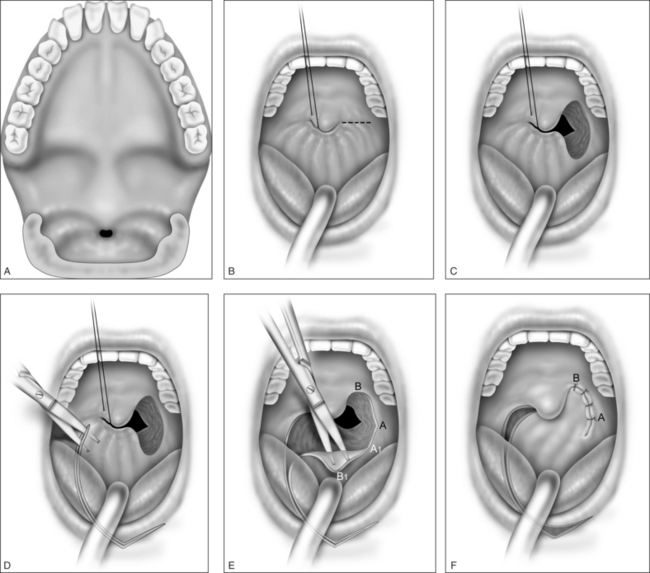
In mild cases, lysis of the scar with some combination of steroid injection, topical application of mitomycin C, and nasopharyngeal stenting may be sufficient to re-establish adequacy of the nasopharyngeal airway. However, frequently simple release of the scarred area results in recurrence, and treatment must therefore include the movement of fresh, well-vascularized tissue to cover the denuded bed. A variety of techniques has been recommended, including Z-plasty, laterally based pharyngeal flaps, other advancement and rotation flaps, and radial forearm and jejunal free flaps. In most cases, the unilateral laterally based pharyngeal flap described by Cotton14 results in an adequate nasopharyngeal airway without restenosis. The procedure is as follows (Fig. 63.3).
3.2.3 ADENOTONSILLAR HYPERPLASIA AND OROPHARYNGEAL OBSTRUCTION
Adenotonsillectomy is generally the first intervention considered for most patients with SRBD, providing they have at least mild adenotonsillar hyperplasia. Improvements in snoring and polysomnography may be anticipated postoperatively in such patients, including those in whom obesity exacerbates their airway obstruction.15–18 Improvement following adenotonsillar surgery has also been demonstrated in children with preoperative enuresis, orthodontic abnormalities, and behavioral issues prior to surgery. Validated surveys suggest an overall improvement in quality of life after adenotonsillectomy.19,20
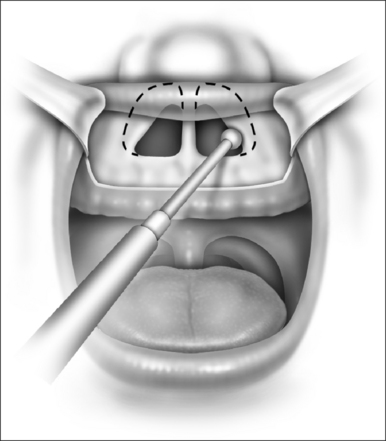
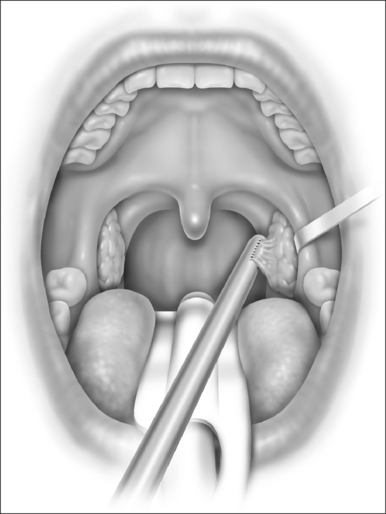
With the report by Krespi and Ling of serial tonsillectomy with CO2 laser in the outpatient setting came the notion that partial tonsillectomy was safe and less painful than traditional tonsillectomy for patients with tonsil hyperplasia.21 It is theorized that the exposure of muscle resulting from removal of the tonsil capsule is the cause of pain associated with tonsillectomy, and that leaving some small portion of the tonsil behind may vastly diminish this sequela.22 Proposed many years ago, the technique had been abandoned due to the risk of tonsil regrowth at a time when most such procedures were performed for recurring infection. Initially studied using the CO2 laser, intracapsular tonsillectomy is now more commonly performed using the microdebrider given its greater efficiency and lower cost (Fig. 63.4). Randomized studies comparing microdebrider intracapsular tonsillectomy to complete electrocautery tonsillectomy generally suggest a modest advantage in pain reduction, particularly otalgia, and return to normal activity; other outcomes yielded less consistent results.23–26 The procedure involves a slight increase in duration and blood loss. Devices for traditional tonsillectomy developed over the last 20 years, such as the harmonic scalpel (Ethicon Endo-Surgery; Cincinnati, OH), radiofrequency devices (Somnoplasty, Somnus Medical Technologies, Sunnyvale, CA), and Coblation® (Fig. 63.5; ArthroCare Corp., Sunnyvale, CA) have been studied in small trials that suggest modest reduction in pain compared with electrocautery, with further decrease in postoperative pain when the tonsil is reduced rather than excised.
< div class='tao-gold-member'>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses